Changes in the US Pharmacopoeia <643>
What are the impacts on TOC determination in the pharmaceutical industry?
 TOC-LCSH
TOC-LCSH
In 1996, the US Pharmacopoeia has introduced the TOC parameter for the determination of impurities in purified water and water for injections. For other waters used in the pharmaceutical industry, the wet-chemical potassium permanganate test continued to be used. Meanwhile however, TOC determination has proven to be so effective that it now replaces the wet-chemical test.
In the current version of the UPS <643> (USP 36-NF 31) a distinction is made between ‘bulk water’ and ‘sterile water.’ The chapter ‘Bulk Water’ includes purified waters that are to be used right away as purified water, water for injection, water for hemodialysis and as condensate of pure steam. The following known conditions apply to TOC determinations:
- Limit of detection of the TOC system used: < 0.05 mg/L C
- Blank water for the preparation of standards, rw: max. 0.1 mg/L C
- Standard solution (sucrose), rs: 0.5 mg/L C
- System suitability solution (benzoquinone), rss: 0.5 mg/L C
- Permitted response: 85 – 115 %
- Limit response for the waters, ru: < (rs-rw)
The chapter ‘Sterile Water’ is new. It includes sterile purified water, sterile water for injections, sterile water for irrigation and sterile water for inhalation. Sterile water can be stored in various packaging configurations.
In comparison to bulk water, however, other conditions for TOC determination apply:
- Limit of detection of the TOC system used: < 0.1 mg/L C
- Blank water for the preparation of standards, rw: max. 0.1 mg/L C
- Standard solution (sucrose), rs: 8 mg/L C
- System suitability solution (benzoquinone), rss: 8 mg/L C
- Permitted response: 85 – 115 %
- Limit response for the waters, ru: < (rs-rw)
Impact of the new determination
The present requirements of the UPS <643> (bulk water) are consistent with the requirements of the European Pharmacopoeia (limit of detection, concentration of the standard solution (sucrose) and system suitability solution (benzoquinone and response). Validation of the TOC system for both determinations is therefore sufficient. In accordance with the new USP <643>, the implementation of a system suitability test using higher concentrations is required. For users of Shimadzu’s TOC systems, this just means the creation of an additional calibration curve (sucrose, 8 mg/mL, see figure 1) and control sample (benzoquinone, 8 mg/mL, see figure 2) as well as extension of the current validation process with these data. Additional modifications of the TOC system are not necessary.
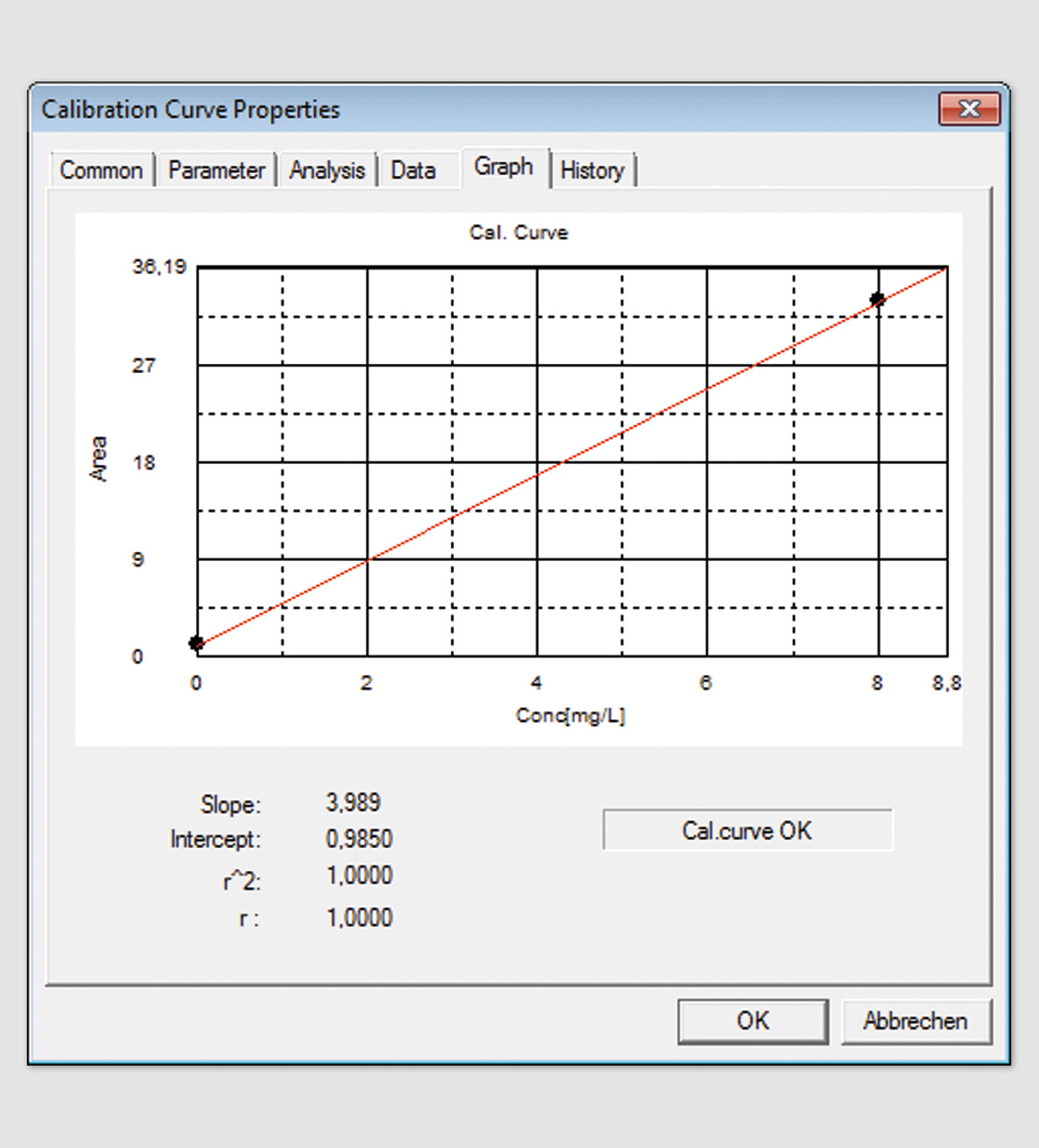 Figure 1: Calibration curve of sucrose, 8 mg/L
Figure 1: Calibration curve of sucrose, 8 mg/L
TOC determination in ultrapure water
Two oxidation techniques are now commonly used in TOC analysis: catalytic combustion and wet-chemical oxidation. In catalytic combustion, carbon compounds are converted to CO2 using high temperatures and a catalyst, with subsequent detection of the resulting CO2 using an NDIR detector. Wet-chemical oxidation uses a combination of UV irradiation and persulfate oxidation. Both methods are suitable for TOC determination in ultrapure water.
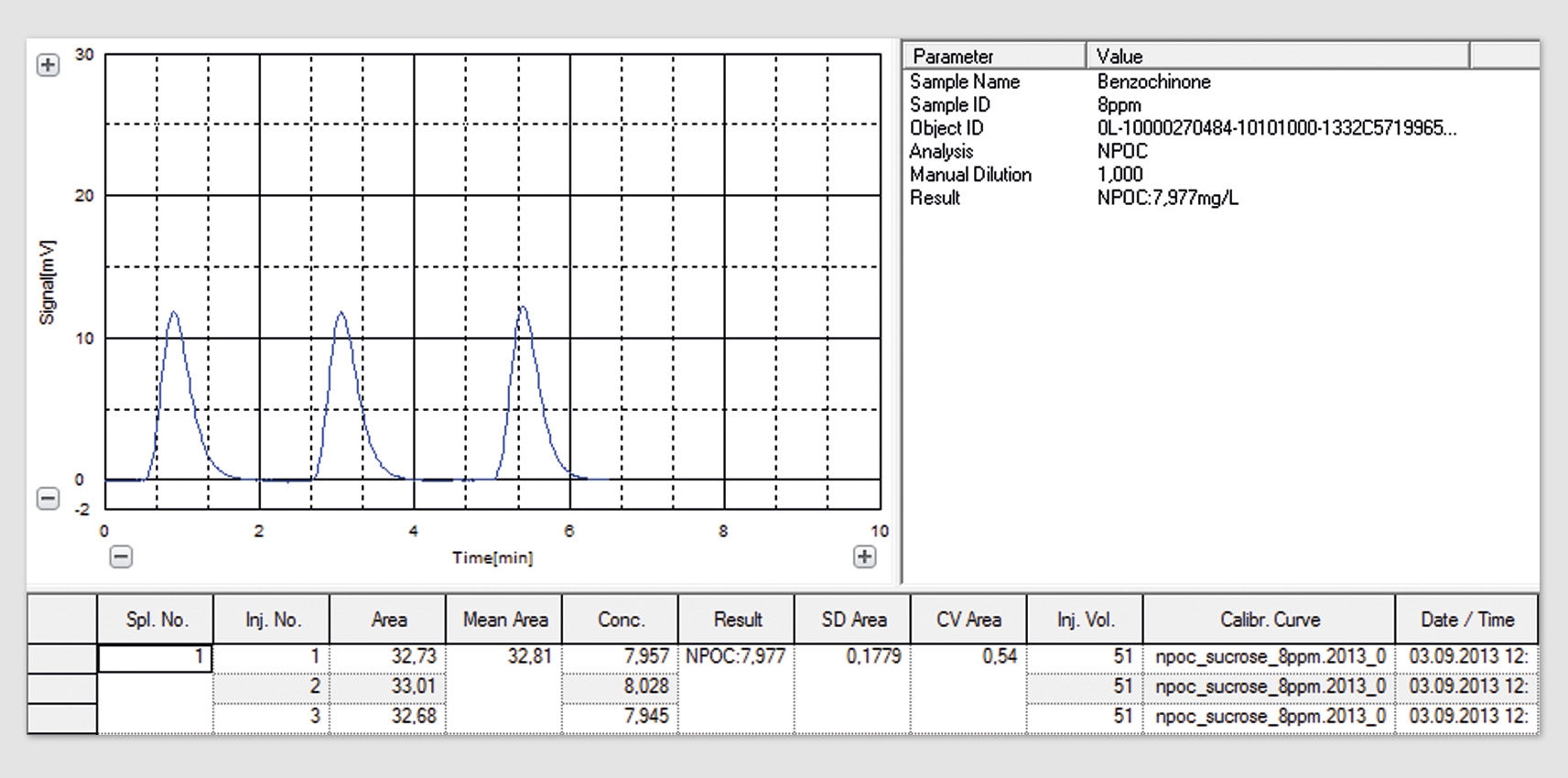 Figure 2: Graphical representation of benzoquinone peaks, 8 mg/L
Figure 2: Graphical representation of benzoquinone peaks, 8 mg/L
Shimadzu TOC system
Shimadzu offers two systems that are ideally suitable for TOC determination in ultrapure water. The TOC-VWP/WS uses wet-chemical oxidation, whereas the TOC-LCPH uses catalytic combustion at 680 °C. With their wide measuring range of 0.5 mg/L up to 30,000 mg/L, the instruments support any application – from ultrapure water (for instance in cleaning validation) to highly polluted waters (such as waste-waters).
TOC-L series – catalytic oxidation at 680 °C
The ISP (Integrated Sample Pretreatment) module for the TOC-L series significantly reduces the user’s workload as the instrument carries out dilution, acidification and sparging. The fully automated dilution from 4 µg/L up to 30,000 mg/L extends the measuring range.
In addition, the combustion method can be used in combination with the TNM-L module, whereby a single injection is sufficient for simultaneous determination of the total bound nitrogen (simultaneous TOC/TNb determination). The EN-compliant determination via chemiluminescence detection is applied. Catalytic combustion is carried out at 720 °C. Simultaneous TOC/TNb determination is highly suitable for cleaning validation, as it enables a differential determination between the cleaning agent and the product.
TOC-V series – wet-chemical oxidation
The key technique of the TOC-VWP series is the powerful oxidation via the combination of sodium persulfate and UV oxidation at 80 °C. As a persulfate solution is used for the determination, it is important that it does not contain any contaminations that could distort the actual measuring value. The TOC-VWP features an automatic reagent preparation function that eliminates possible contaminations of the persulfate solution. This ensures that the average TOC value truly originates from the sample – and not from the reagent solution used.
The large injection volume (up to 20.4 mL) in combination with the highly sensitive NDIR detector, leads to an extremely low limit of detection and excellent reproducibility in the lower ppb range. The TOC-VWP/WS is therefore highly suitable for TOC determination in the ultra-trace range.
Conclusions
Both types of instrument with their different oxidation methods can be used for TOC determination in accordance with the new United States Pharmacopoeia (USP <643>) and the European Pharmacopoeia (EP 2.2.44). The advantage of the combustion method is its high oxidation potential, especially for samples containing particulate matter. Moreover, simultaneous TOC/TNb measurements can be carried out, leading to a higher information content of the analysis. The advantage of wet-chemical oxidation is its very high injection volume, which leads to higher sensitivity and therefore enables high precision measurements in the lower ppb range.