Lighting up
Determination of quantum efficiency of fluorescence standards with high quality

One of our first experiences with materials lighting up at night is definitely the glowing dial of a wristwatch. A wide variety of materials exhibit this property in which energy supplied from an external source leads to the emission of light (fluorescence or phosphorescence). This physical principle of fluorescence is used in the development and quality control of lighting systems, displays or monitors, as well as in biotechnology and medicine. For these applications, modern instrumental analysis makes use of reliable and precise measurement technologies to characterize this fluorescence.
In fluorescence spectroscopy, there is an interdependence between instrument technology and the wavelength of the light. Modern instruments can perform computational spectra correction in order to reliably compare different instruments. Such a correction takes into account the characteristics of the emission optics as well as the excitation optics. The spectra corrected for these characteristics are subsequently used to determine the so-called quantum yield of the fluorescing materials (fluorophores). The quantum yield describes the probability of how effectively a material can convert the irradiated energy by the excitation light into the emitted fluorescent light.
Quantum yield: relative and absolute method
In the past, this determination was carried out using a relative method, in which a known reference standard was measured and its fluorescence intensity was then compared to that of a sample. For unknown compounds, however, these quantum yields must be determined in a different way via an absolute method – known as ‘quantum efficiency’ (QE).
In this case, the quantum yield is calculated directly from a spectrum measured from the fluorescent substance using an integrating sphere, which effectively collects all fluorescence quanta and passes these to the emission detector. Figure 1 illustrates the approach and mathematics for the determination of the quantum efficiency. In 2011, IUPAC published a technical report [1] in which the quantum yields of various fluorescence standards were compared based on the existing literature. As a small set of well-established standards, quinine sulfate, fluorescein and rhodamine 6G are listed. In addition, the review also includes 9,10-diphenyl anthracene, beta-carboline, (norharmane), harmaline, rhodamine 101 and cresyl violet as well as 28 other frequently used substances [1].
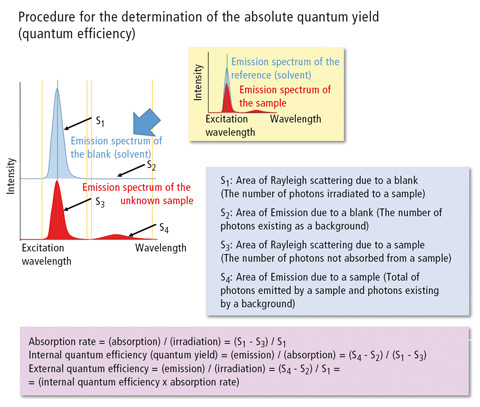 Figure 1: Determination of the absolute quantum yield
Figure 1: Determination of the absolute quantum yield
Fluorescence standards dependent on solution conditions
When considering the selection of the standard, the conditions of the solution in which the standards are prepared are important. Solvent concentration, pH value in aqueous solution and also temperature must be taken into account. Different solvents used to prepare the fluorescent standards lead to different quantum yields.
In addition to these aspects, the IUPAC report also discusses the measurement technology applied. Two variations are the rule:
- relative measurement of quantum yield using a cuvette in a standard sample holder of a fluorescence spectrometer.
- absolute measurement of quantum efficiency using an integrating sphere.
The present application not only demonstrates the absolute determination of the quantum efficiency of three fluorophores using Shimadzu’s RF-6000 with integrating sphere (figure 2) but also the good correlation of these results with corresponding literature values. In this application, the quantum efficiencies of fluorescein, quinine sulfate and tryptophan were determined as examples.
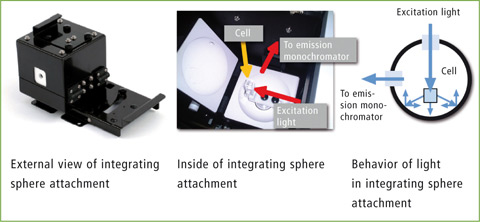 Figure 2: Integrating sphere for the RF-6000; left: integrating sphere; middle: cuvette in the opened sphere; right: graphical representation of the light path of excitation and emission within the sphere.
Figure 2: Integrating sphere for the RF-6000; left: integrating sphere; middle: cuvette in the opened sphere; right: graphical representation of the light path of excitation and emission within the sphere.
Carrying out the measurement
Prior to measurement, the appropriate concentration of the solution is adjusted and the diluted fluorophore is transferred to a cuvette with four polished sides. This cuvette is placed into the integrating sphere of the RF-6000 and measurement is subsequently started.
The measurement parameters selected in the LabSolutions RF software are listed in table 1. A measurement was carried out with an average speed of 600 nm/min. The instrument was set to a slit width of 5 nm for excitation as well as for emission. Detector sensitivity was set to low.
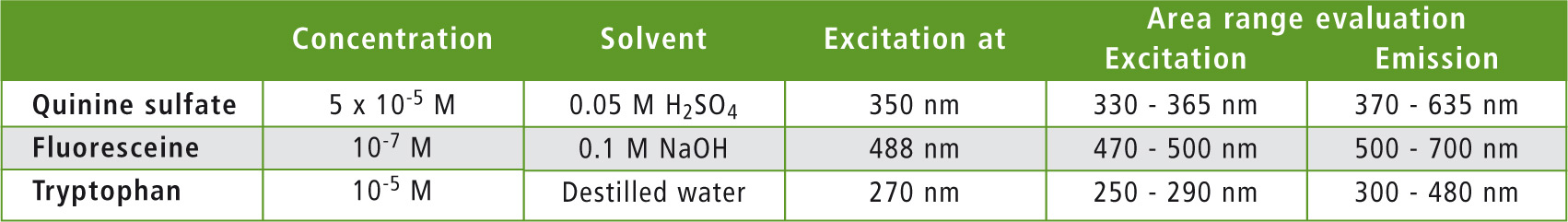 Table 1: Conditions for evaluation of the measured spectra for quantum efficiency determination
Table 1: Conditions for evaluation of the measured spectra for quantum efficiency determination
The ‘Quantum Efficiency’ function of the LabSolutions RF software was used to evaluate quantum efficiency. The areas used for signal integration are listed in table 1. As an example, the quantum efficiency measurement of quinine sulfate is shown in figure 3. Table 2 compares the measured absolute quantum efficiency with the data obtained from the literature.
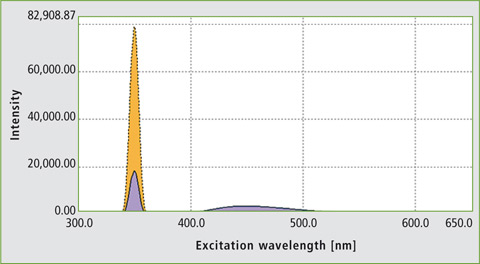 Figure 3: Determination of the quantum efficiency of a quinine solution at a concentration of 5 x 10-5 M; QE was calculated as 0.5898
Figure 3: Determination of the quantum efficiency of a quinine solution at a concentration of 5 x 10-5 M; QE was calculated as 0.5898
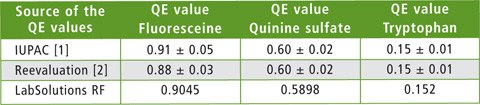 Table 2: Comparison of absolute quantum yields (QE, quantum efficiency) for fluorescein, quinine sulfate and tryptophan measured with an integrating sphere (data obtained from the literature) with quantum yields obtained using the RF-6000 with integrating sphere
Table 2: Comparison of absolute quantum yields (QE, quantum efficiency) for fluorescein, quinine sulfate and tryptophan measured with an integrating sphere (data obtained from the literature) with quantum yields obtained using the RF-6000 with integrating sphere
All three QE values of the fluorescence standard examined lie exactly within the range of the described target value.
Conclusion
The RF-6000 is excellently suited to quantum yield measurements. With its high signal-to-noise ratio in combination with its high speed and quantum yield/efficiency software, absolute quantum yields can be determined quickly and accurately.
Literature
[1] Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report)* / Albert M. Brouwer; Universiteit van Amsterdam, P.O. Box 94157, 1090 GD Amsterdam, The Netherlands, Pure Appl. Chem., Vol. 83, No. 12, pp. 2213-2228, 2011; doi: 10.1351/PAC-REP-10-09-31; © 2011 IUPAC, Publication date (Web): 31 August 2011
[2] Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector / Kengo Suzuki, Atsushi Kobayashi, Shigeo Kaneko, Kazuyuki Takehira, Toshitada Yo-shihara, Hitoshi Ishida, Yoshimi Shiina, Shigero Oishic and Seiji Tobita; Phys. Chem. Chem. Phys., 2009, 11, 9850-9860; DOI: 10.1039/b912178a