Compliance made easy
TOC determination in a regulated laboratory environment

Since the American and European Pharmacopeia requirement of TOC determination for testing ultrapure water (water for injection, highly purified water), TOC measuring systems have been used extensively in the pharmaceutical industry. In addition to the ultrapure water analysis application, TOC determination is used for cleaning validation and in the testing of plastic packaging and its materials. This is why TOC instruments are used in a regulated laboratory environment and why these systems are subject to various regulations that apply particularly to instrument software, which is focused in this article.
GLP/GMP refers to the organizational process
One of these regulations is “Good Laboratory Practice” (GLP), which refers to the organizational process and the conditions involved in the planning, implementation and monitoring of laboratory studies and testing as well as the recording and reporting of analytical results. This means that the instrument software authorizes access to the system and fully documents data and parameter changes as well as events and results.
 Figure 1: LabSolutions splash screen
Figure 1: LabSolutions splash screen
FDA 21 CFR Part 11 covers the use of electronic records
In 1997, the American Food and Drug Administration initially developed guidelines on the use of electronic records and electronic signatures, in order to drastically reduce the necessity for paperwork. As electronic information is easier to falsify, 21 CFR Part 11 defined criteria under which applications and documentation must be digitally filed and electronic signatures can be recognized. This is to guarantee that electronic documents are as trustworthy and reliable as paper records.
Control mechanisms and directions on procedures must safeguard data authenticity – and if necessary also data integrity. Most Part 11 regulations involve safety measures against illegal system access, user management, data security, data archiving and the electronic signatures themselves. The TOC-Control L software running the TOC-L series provides full support for complying with CFR 21 Part 11, while still remaining extremely user-friendly.
TOC-Control L software supports analysis work and data reliability assurance
Already during software installation, the operating criteria of the software are defined. The parameters selected cannot be deactivated afterwards. The TOC-Control L software provides a wealth of functions to fully support analysis work and to support data reliability assurance.
User administration
Software utilization is enabled via user access rights. It offers individual accounts on four different levels, each protected through own passwords. The administrator can change access rights for each user. TOC-Control L allows changing of login during ongoing operations. This is especially important for laboratories working in multiple shifts. User administration is carried out in an external management software, where parameters for passwords like minimum length, complexity and validity period can be specified. To prevent illegal access, it is possible to set lockout functions and automatic email transmissions in case of specified events.
Audit Trail for documentation purposes
All software operations are stored automatically in the audit trail (figure 2) which operates in the background. Entering of a comment can be set as mandatory when parameters are modified. Data storage takes place in a relational database. Through targeted queries specific operations can easily be referred to. It is possible to sort data according to time periods as well as user names or topics such as maintenance, administration or sample measurement. It is possible to manually enter maintenance operations that are not recognized by the software (e.g. replacement of the catalyst).
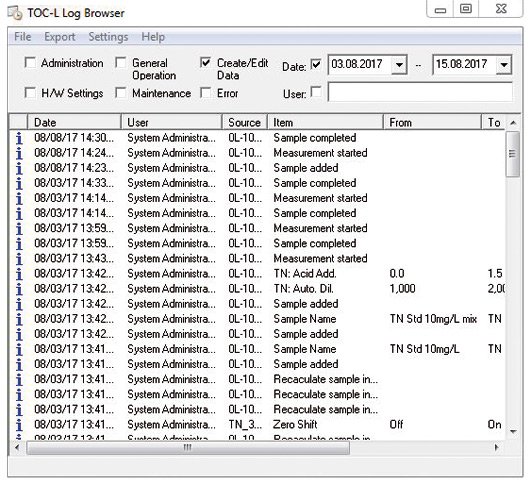 Figure 2: Audit trail
Figure 2: Audit trail
Raw data management provides data acquisition security
In a regulated laboratory environment, data acquisition security is particularly important. Data acquired may not be altered by users not recognized by the software. TOC determination starts with peak integration, which cannot be influenced by the user. The peak area obtained is subsequently converted into a concentration value via a calibration curve. Recalculation applying a different calibration curve is possible, if permitted by the administrator.
All data is stored automatically, and the respective data files contain all important information: the system utilized, the user, the methods and calibration curves applied, the measured results (areas and concentrations) and the peak profile. For data protection and backup, these data files can be exported into a database directly after creation. Output is possible in human-readable form in .txt or .pdf file format.
LabSolutions: cross-functional data management
For solid data security and improved management efficiency, Shimadzu offers the LabSolutions software platform. LabSolutions is a cross-functional data management concept for FDA 21 CFR Part 11 compliance and is applicable across the entire laboratory. Using LabSolutions, all data generated by various Shimadzu systems can be administered, signed and archived centrally in a relational database. This enormous amount of data can be structured project-wise to enable easy search and management.
The possibility to create customizable multi-data reports from different types of analysis simplifies reporting to decision-makers and contractors. Support for computerized system validation (CSV) and predefined validation templates within TOC-Control L, facilitate easy system validation while minimizing possibilities of error. The LabSolutions platform can be set up on a single-PC base (LabSolutions DB) for smaller laboratories or in a company-wide client server network system (LabSolutions CS).
Support for TOC determination according to EP 2.2.44
The TOC-Control L software simplifies the implementation of tests with integrated templates for the creation of calibration curves (figure 3) and measurement of control samples.
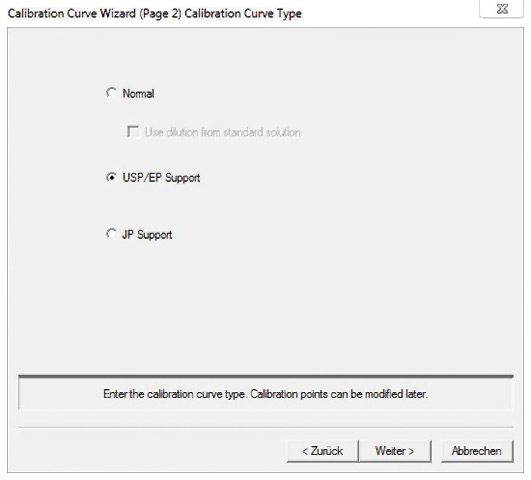 Figure 3: Template for EP/USP calibration curve
Figure 3: Template for EP/USP calibration curve
The system suitability test is predefined in a method template (figure 5). Following measurement of the control sample (benzoquinone), recovery is calculated automatically, compared with the predetermined limits (85-115 %) and documented (figure 4).
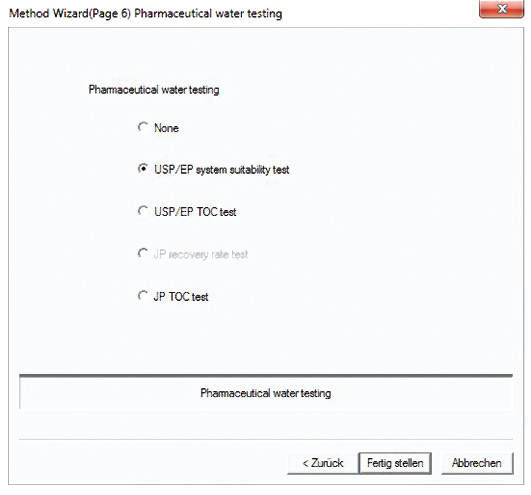 Figure 4: Executed system suitability test with automatic calculation
Figure 4: Executed system suitability test with automatic calculation
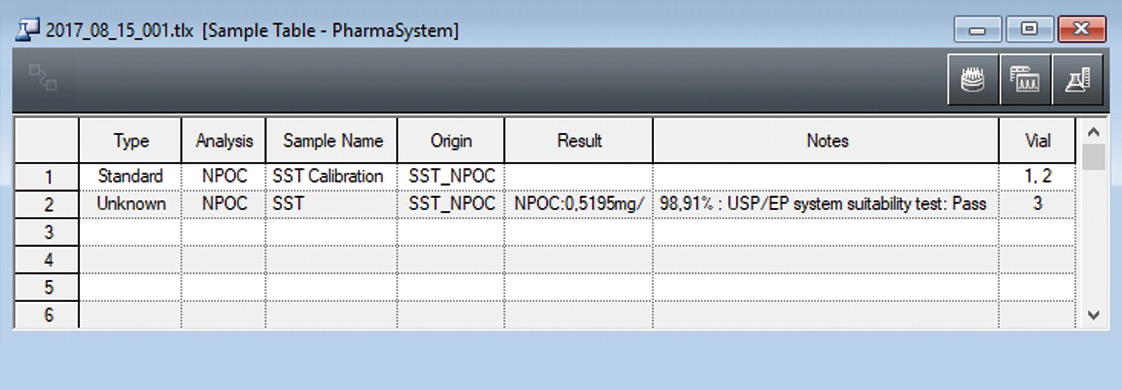 Figure 5: Template for the system suitability test
Figure 5: Template for the system suitability test
Conclusion
The TOC-Control L software combines all necessary functions for secure data handling while supporting user-friendly compliance with existing regulations in the pharmaceutical industry.