How X-ray fluorescence can support consumer prices stability
Aluminum fluoride all-in-one analysis with EDX-8000P
 Figure 1: Aluminum cookware: the easiest way to cook and transport various food dishes
Figure 1: Aluminum cookware: the easiest way to cook and transport various food dishes
Nowadays, aluminum as a chemical element plays an important role in everyday life. Due to its low weight and good thermal conduction, aluminum and its alloys are the basis for modern aviation industry, aerospace engineering, production of automobiles, rolling stock for high speed trains, sea and river vessels…
Moreover, this element is extremely popular in the production of cookware or foils used for packaging in the food industry.
Aluminum is produced by electrolysis of alumina dissolved in a molten cryolite bath. The process of electrolysis requires a huge amount of electricity. Some industrial processes using aluminum fluoride AlF3 (approximately 5 – 15 %) and other additives have been developed to decrease the melting point to 930 – 950 °C and to increase conductivity of the electrolyte solution. This reduces energy consumption and greatly decreases the cost of the aluminum.
Before being used as an additive, aluminum fluoride has to be analyzed in order to check the content of the main component. At the same time, the main impurity (aluminum oxide) as well as harmful elements such as silicon, phosphorus and iron are examined in order to prevent main product contamination (metallic aluminum).
AlF3 quality analysis: a constant challenge for laboratories
According to the Russian GOST regulation (national standard of the Russian Federation and CIS countries), standard chemical analysis of aluminum fluoride includes many time-consuming sample preparation steps including fusion and dissolution. In addition, different analysis methods are used for each compound: titration for quantitative analysis of AlF3, free aluminum oxide (Al2O3) determination by trilonometric measurement of aluminum, and photometric analysis of silicon, phosphorus and iron oxides (SiO2, Fe2O3 and P2O5). So, complete analysis of one sample (including sample preparation) takes more than twelve hours and requires many chemical reagents and devices.
Use of the EDX-8000P Energy Dispersive X-ray fluorescence spectrometer appears to offer a valuable alternative way to simplify and dramatically decrease analysis time. This spectrometer is in fact able to analyze all elements from carbon up to uranium in a simultaneous measurement. Moreover, target concentration ranges are compatible with the EDX spectrometer sensitivity. The EDX-8000P is BfS (safety standards of the German Federal Institute for Radiation Safety) type approval certified.
 Figure 2: Energy dispersive X-ray fluorescence spectrometer EDX-8000P
Figure 2: Energy dispersive X-ray fluorescence spectrometer EDX-8000P
New fast and easy X-ray fluorescence method for AlF3 analysis
Samples of AlF3 were prepared for analysis by grinding in the mixer mill and pressing in the pellet press. The same sample preparation is used for single AlF3 standard sample with a known content of the main component and Al2O3. Calibration samples for analysis of SiO2, P2O5 and Fe2O3 were prepared by adding known amounts of element oxides to AlF3 standard sample and subsequent mixing, grinding and pressing.
A routine measurement procedure of unknown sample by Fundamental Parameters (FP) method is included in the standard spectrometer software (PCEDX). Nevertheless, a single standard sample was used to perform methods calibration in order to improve accuracy of results. Aluminum fluoride content was calculated on the basis of fluorine element concentration. Aluminum oxide Al2O3 concentration was then evaluated by the aluminum peak after aluminum fluoride subtraction. Results of X-ray and chemical (titration and photometry) analysis obtained for fluorine and aluminum are given in table 1.
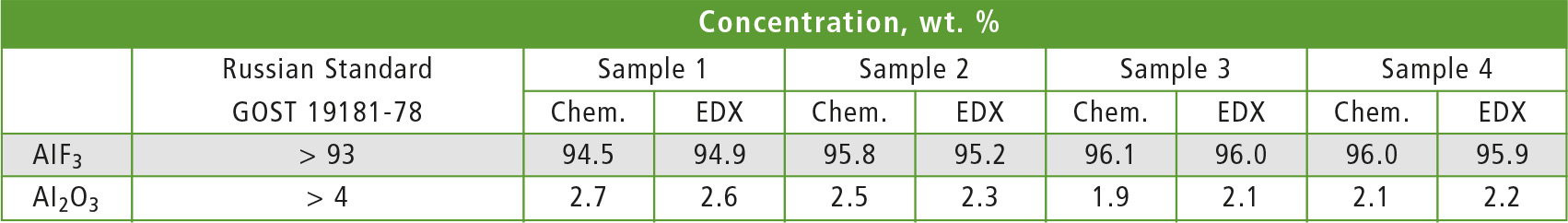 Table 1: Comparison of results of main component and Al203 in AlF3 analysis by chemical methods (Chem.) and EDX-8000P
Table 1: Comparison of results of main component and Al203 in AlF3 analysis by chemical methods (Chem.) and EDX-8000P
The other contaminants (silicon, phosphorus and iron oxides) were analyzed using calibration curve method with corresponding element specific Ka lines. Calibration curves for each oxide are shown in figure 3. The results of X-ray and chemical analyses are given in table 2.
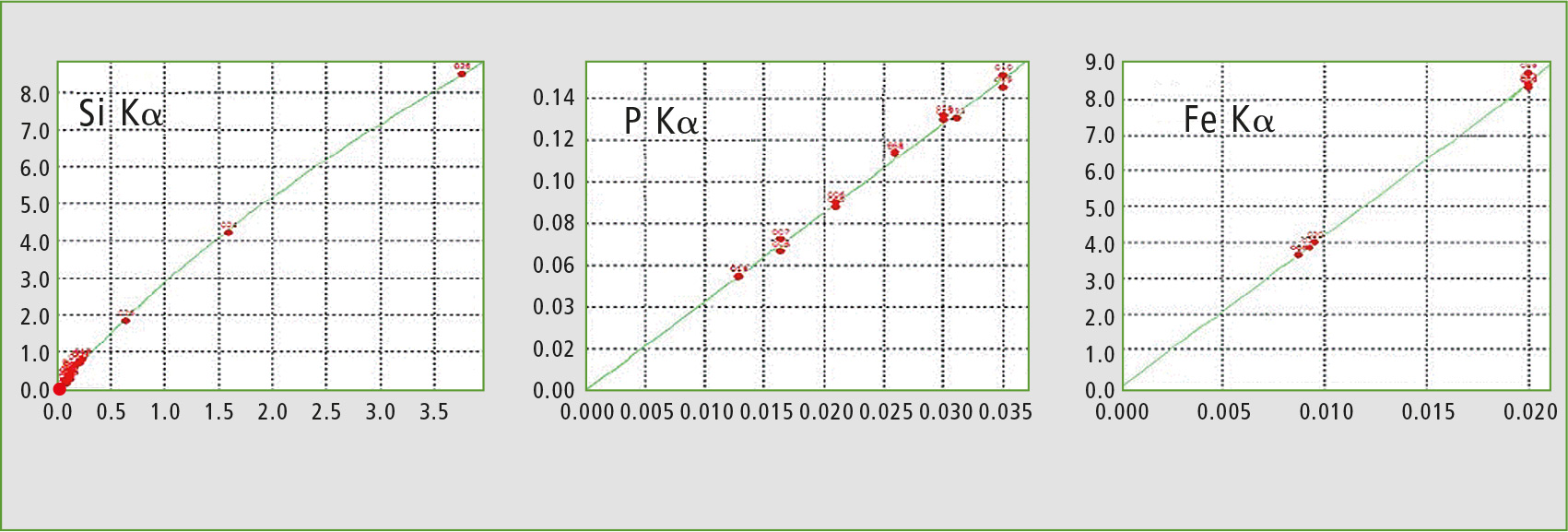 Figure 3: Calibration curves obtained for each targeted contaminant
Figure 3: Calibration curves obtained for each targeted contaminant
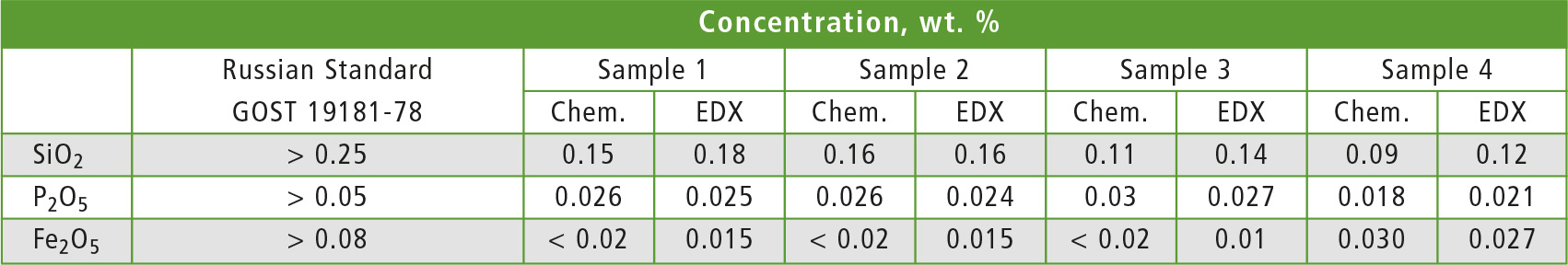 Table 2: Comparison of results for SiO2, P2O5 and Fe2O3 analysis by chemical methods (Chem.) and EDX-8000P
Table 2: Comparison of results for SiO2, P2O5 and Fe2O3 analysis by chemical methods (Chem.) and EDX-8000P
Total analysis time of all elements including sample preparation was only approximately thirty minutes.
Conclusion
All EDX results shown in tables 1 and 2 showed excellent correlation with official regulation analysis methods results. This demonstrates that the EDX-8000P analysis procedure is a valuable alternative to traditional time-consuming methods. By decreasing analysis time and chemical product consumption, this new strategy can contribute to reduced aluminum material prices and in the end to price stability of consumer prices for food packaging, convenience foods and freshly prepared meals on wheels.
Acknowledgements
We gratefully acknowledge the valuable advice and assistance in EDX analysis of our colleagues from the accredited Analit Company (St. Petersburg, Russia) laboratory.