DOSIURA™, a new solution for DPD deficiency screening
Uracilemia measurement by LC-MS/MS as an accurate approach
Sibylle Collard, Julia Petit, Mikael Levi, Alsachim
Screening for DPD (dihydropyrimidine dehydrogenase) deficiency in patients needing fluoropyrimidine-based chemotherapy is becoming a strong recommendation – if not mandatory – by health authorities in Europe in order to reduce the risk of severe drug toxicities that these treatments can cause. Indirect phenotyping of DPD deficiency can be performed by measuring the level of uracil and dihydrouracil in plasma. To facilitate the detection of DPD deficiency, the DOSIURA™ reagent kit offers a turnkey analytical solution with all the needed reagents for the precise quantification of these compounds by LC-MS/MS.
Fluoropyrimidines, a group of anticancer drugs including 5-fluorouracil (5-FU), are widely used in the treatment of solid tumors such as colorectal, gastric, ENT and breast cancers. However, fluoropyrimidine-based chemotherapies could expose patients to severe side effects, occurring in one in five cases, and can cause severe toxicities (grades 3–4 according to the WHO classification of chemotherapy toxicities) and rarely but occasionally deaths (incidence between 0.1 and 1 %).[1]
These toxicities may be linked to a deficiency in the activity of the main enzyme involved in 5-FU elimination, dihydropyrimidine dehydrogenase (DPD). (Figure 1)
DPD is an enzyme that assists the body in the catabolism of endogenous pyrimidines (uracil and thymine) and fluorinated pyrimidines such as 5-FU. With low activity or without enough DPD enzyme, these chemotherapy drugs accumulate in the body and cause more serious side effects than anticipated. Patients with a significant DPD deficiency receiving 5-FU treatment have an increased risk of toxicity. DPD activity varies from one individual to another, so it’s essential to know whether a deficiency exists before starting treatment.
Today, many health regulatory authorities in Europe, and even worldwide, tend to recommend or require systematic DPD deficiency assessment in patients receiving fluoropyrimidine-based chemotherapy.
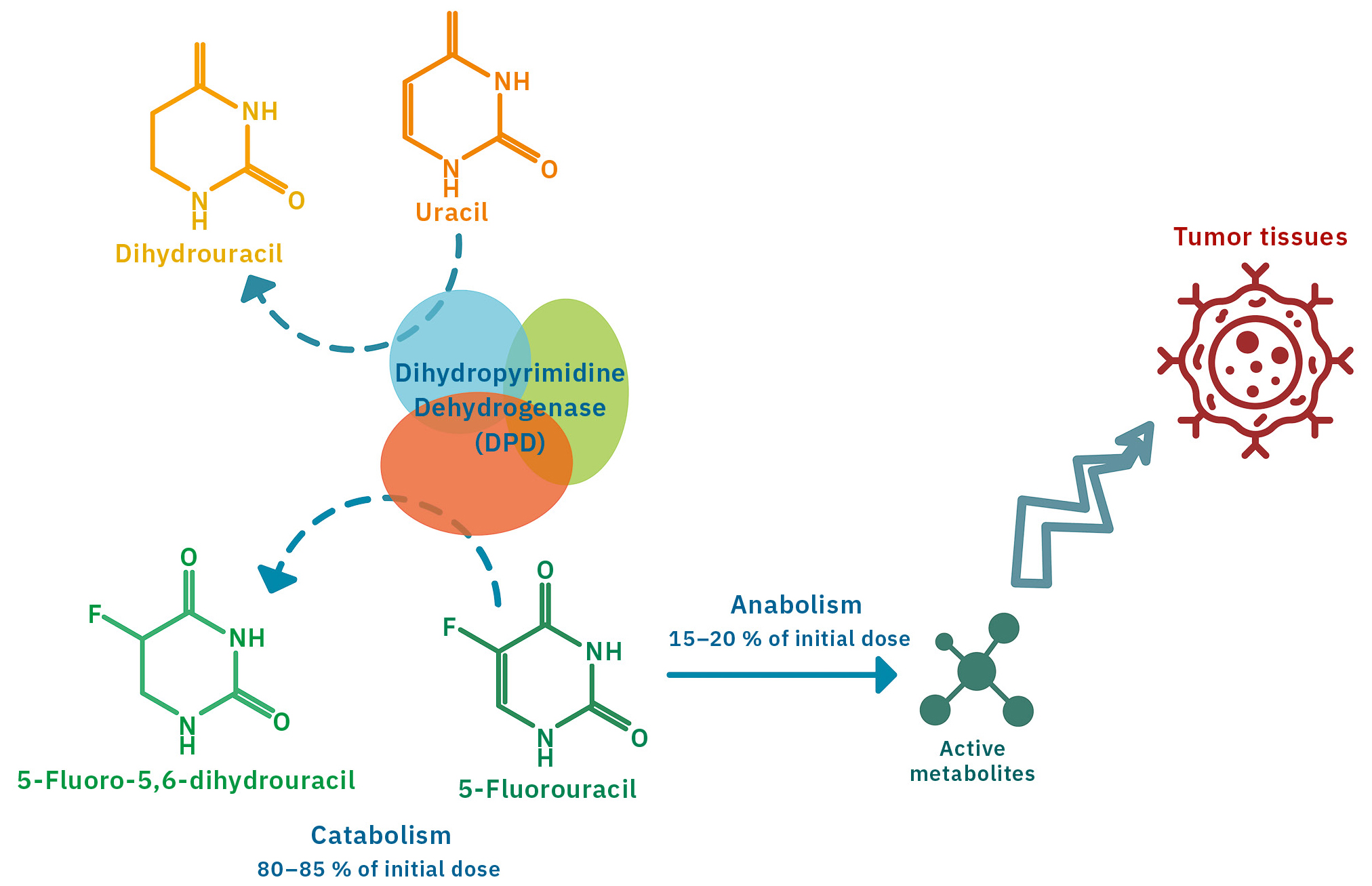
Testing for DPD deficiency by genotyping or phenotyping
Genotyping is the search for variants of the DPYD gene encoding the DPD enzyme, while phenotyping involves direct or indirect measurement of the enzyme’s activity.
Genotyping is currently based on the search for four variants: DPYD*2A, DPYD*13, c.2846A>T and HapB3. It can detect deficiencies but does not cover all populations,[1] and studies highlighted many toxicities not explained by mutation on these four variants.
The alternative method, phenotyping, involves direct measurement of DPD activity in PBMCs (peripheral blood mononuclear cells) or indirect measurement by quantification of uracil and dihydrouracil levels in plasma. However, direct measurement of the enzyme activity is very complex and time-consuming, thus, indirect phenotyping is preferred. Plasma concentrations of these two compounds are determined by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
DOSIURA™, a turnkey solution for rapid quantification of uracil and dihydrouracil
Alsachim, a Shimadzu Group company, has developed DOSIURA™, a turnkey solution for the quantification of uracil and dihydrouracil in plasma, enabling rapid assessment of DPD deficiency. This innovative reagent suite and its analytical method provide an answer to many of the problems faced by laboratories and hospitals in the phenotyping of DPD deficiency.
Following recommendations for preventive screening for DPD from health authorities like the European Medicines Evaluation Agency (EMEA) from 2020,[2] the ANSM in France and the MHRA in the UK, hospitals and biomedical laboratories are facing a double challenge: an increased demand for DPD deficiency analyses [3] and the need to develop long and highly complex sample preparation and analytical methods to perform these analyses.
DOSIURA™, the only one of its kind on the market, offers the required reagents and a rapid analytical method for DPD deficiency testing
Based on the indirect phenotyping approach, more reliable and reproducible, and the LC-MS/MS method offering simultaneous quantification of several compounds, the kit enables rapid measurement of uracil and dihydrouracil.
DOSIURA™ provides the quality reagents needed for these analyses: calibrants, internal standards (synthesized by Alsachim’s expert chemists) and controls, eliminating the complexity and analytical instability associated with reagent preparation for laboratories. “The controls are prepared in real human plasma and formulated at decision threshold concentrations for DPD deficiency assessment, which facilitates the clinician decision-making process following analysis of the results,” explains Julia Petit, project leader in R&D at Alsachim.
DOSIURA™ is a complete solution including necessary consumables and a robust analytical method:
- 3 sets composed of calibrators with 6 levels and blank, stable-isotope-labeled internal standards and quality controls with 3 levels
- Ready-to-use reagents: mobile phases and cleaning phase, extraction buffer and analytical column
- Sample preparation protocol and analytical parameters are already set.
Uracil and dihydrouracil are polar endogenous compounds with low molecular weights, making method development a real challenge for the R&D team, especially the separation of target compounds from all interfering molecules present in the matrix. “Our objective was to provide our customers with a fast, accurate and robust solution,” says Julia Petit.
The DOSIURA™ solution offers metrological traceability of these compounds and a very fast sample preparation method: the protocol and the reagents provided allow faster sample preparation compared to published methods.[4, 5] „We aimed to offer a complete and rapid solution to tackle the problems that arise in hospitals and laboratories. Screening for DPD deficiency is a major issue, and through DOSIURA™ we are willing to support research into the treatment of cancer patients and to be a key partner for our customers in their daily work.”
Ensuring the safety of cancer treatment
With DPD deficiency screening becoming more widespread in countries around the world and with the entry into force of the IVDR and its requirements in terms of medical devices and standardization of methods, DOSIURA™ can be a major asset for standardizing and routinely analyzing uracil and dihydrouracil concentrations in a rapid fashion.
DOSIURA™ is for research use only, not provided to diagnostic use.
[1] Recherche de déficit en dihydropyrimidine déshydrogenase en vue de prévenir certaines toxicités sévères survenant sous traitement comportant des fluoropyrimidines (5-fluorouracile). Recommandations et référentiels. INCa. HAS. December 2018, updated September 2023.
[2] Fluorouracil and fluorouracil related substances (capecitabine, tegafur and flucytosine) containing medicinal products. Assessment report. EMA/274404/2020.
[3] de With, M., Sadlon, A., Cecchin, E., Haufroid, V., Thomas, F., Joerger, M., van Schaik, R.H.N., Mathijssen, R.H.J., Largiadèr, C.R. ‘The Working Group on the Implementation of DPD-deficiency Testing in Europe’ (Apr 2023). Implementation of dihydropyrimidine dehydrogenase deficiency testing in Europe. ESMO Open. 8 (2): 101197. doi: 10.1016/j.esmoop.2023.101197. Epub 2023 Mar 28. PMID: 36989883. PMCID: PMC10163157.
[4] Tafzi, N., Woillard, J.B., Fleytoux, A., Picard, N., Marquet, P. (Aug 2020). Phenotyping of Uracil and 5-Fluorouracil Metabolism Using LC-MS/MS for Prevention of Toxicity and Dose Adjustment of Fluoropyrimidines. Ther Drug Monit. 42 (4): 540–547. doi: 10.1097/FTD.0000000000000768. PMID: 32384537.
[5] Coudoré, F., Roche, D., Lefeuvre, S., Faussot, D., Billaud, E.M., Loriot, M.A., Beaune, P. (Nov–Dec 2012). Validation of an ultra-high performance liquid chromatography tandem mass spectrometric method for quantifying uracil and 5,6-dihydrouracil in human plasma. J Chromatogr Sci. 50 (10): 877–84. doi: 10.1093/chromsci/bms085. Epub 2012 Jun 11. PMID: 22689904.