Future-proof analysis – UHPLC 3.0
Nexera perfectly serves all needs of the Pharmaceutical Industry
 Figure 1: Nexera System with Rackchanger II for large sample capacity
Figure 1: Nexera System with Rackchanger II for large sample capacity
No matter whether Drug Discovery, Pre-Clinical and Clinical Studies, Active Ingredients or Finished Products – HPLC is the technique of choice in the Pharmaceutical Industry. Depending on the stage, e.g. drug development or production of finished pharmaceuticals, there are many demands on HPLC system properties.
Nowadays, screening for active substances as well as the chemical optimization is unthinkable without the use of LCMS. There are different needs and priorities for the MS detectors applied in this stage, but the requirements for front-end HPLCs are more or less the same for all. The systems are expected to perform high resolution analysis at high speed with zero carry over. Furthermore, they should be capable of handling various plate and vial formats as well as large sample quantities. The systems need to perform consistently with a minimum of variations from system to system so that poor precision does not obstruct data pooling later on. Since sample amounts are often limited, there is a high demand for systems dealing with smallest sample quantities while still providing high sensitivity measurements.
At a later stage, testing for identification, quantification/impurity, stability/content uniformity, dissolution as well as cleaning validation requires different and additional options. HPLC determinations in cleaning validation demand high sensitivity and universal detection using large sample quantities at low concentrations, while HPLC determinations in dissolution testing require specific accurate detection of large and small quantities within a limited time. Quality Control usually calls for precise and robust HPLC systems. High sensitivity is important for stability and impurity testing, since degradation products usually appear in much smaller concentrations.
High system precision enables early trend recognition and intervention before out-of-specification results, thereby offering major advantages over medium-precision HPLC systems.
Whether in drug detection or quality control, the choice of dedicated HPLC systems should not be limited by the software platform controlling the systems and processing the data. In development of the Nexera UHPLC system, Shimadzu not only focussed on high throughput, high resolution capable of handling modern sub 2 µm particle columns with system pressures over the 1,000 bar limit of most current UHPLC systems. Shimadzu also aimed at a versatile system with almost zero carry-over which could be utilized in any area based on its comprehensive pressure flow range and scalable injection volume range. Shimadzu’s Nexera provides a flexible all-round HPLC system with a precision and robustness suited for daily routine analysis, as well as groundbreaking high speed and high resolution separations. Nexera can be controlled by the LabSolutions software and other software platforms such as Waters EmpowerTM, Dionex ChromeleonTM and AB Sciex AnalystTM.
Nexera is a modular and flexible UHPLC system consisting of
- LC-30AD high precision solvent delivery pumps
- fast and flexible SIL-30AC autosampler with lowest carry-over marketwide
- Rackchanger II for extra-large sample quantities
- standard column thermostat handling column switching valves, mixing reactors and large columns as well as the new dedicated high temperature column thermostat allowing column heating up to 150 °C.
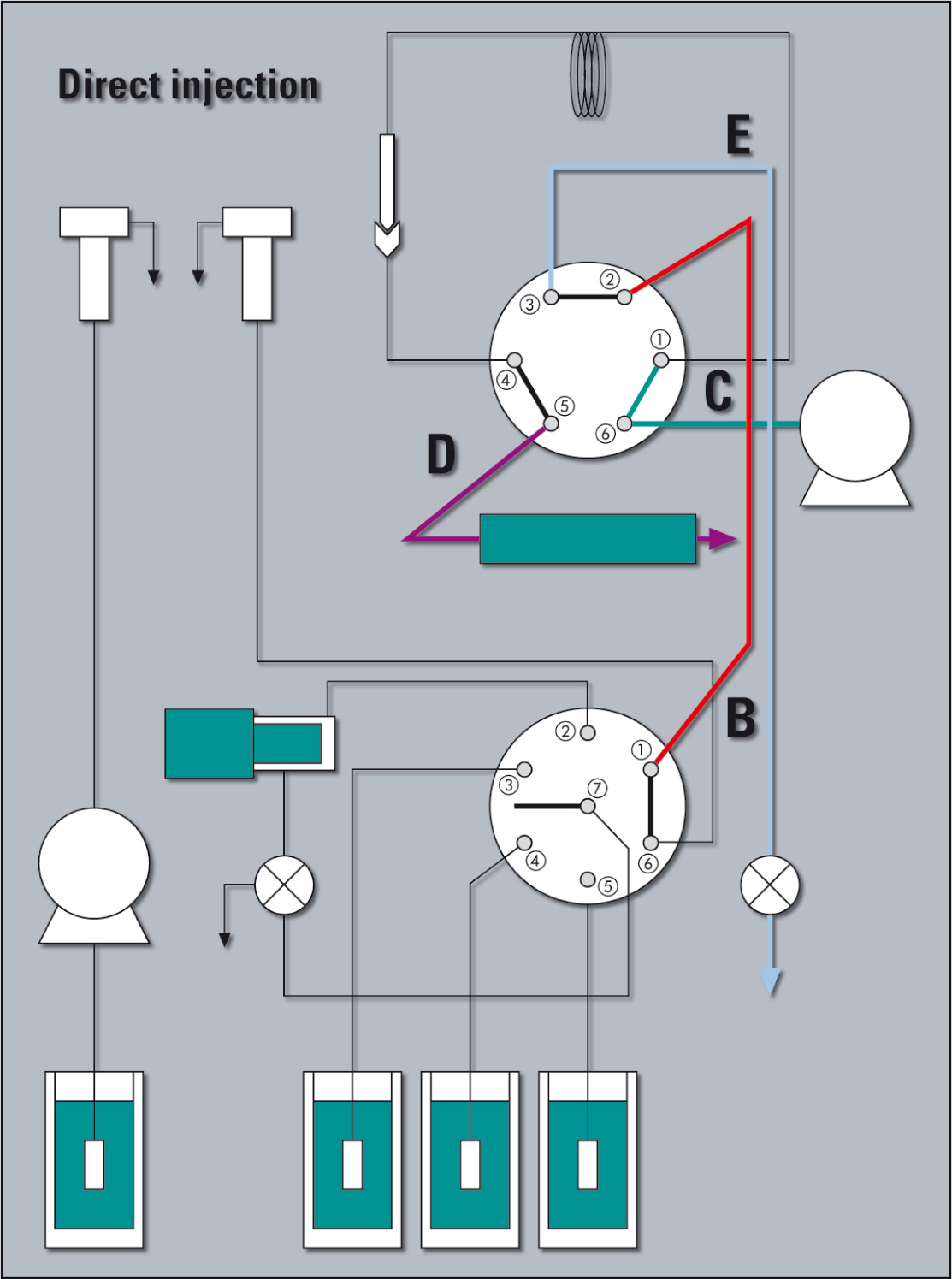 Figure 2: SIL-30AC Flow Line DIIMS. Sample suction line is part of the flow line and the metering pump is isolated via a low pressure valve.
Figure 2: SIL-30AC Flow Line DIIMS. Sample suction line is part of the flow line and the metering pump is isolated via a low pressure valve.
With the low dispersion semi-micro flow cells and the 100 Hz sampling rate, Shimadzu HPLC detectors are fit for high speed / high resolution separations.
In the following text, the main Nexera modules are described in more detail.
The LC-30AD solvent delivery module is a high resolution micro volume dual piston pump bringing new performance in precision and accuracy to modern solvent delivery pumps. Its flow rate range covers 0.0001 mL/min to 10 mL/min with a pressure range up to 130 MPa. The LC-30AD allows setting up of binary or ternary high pressure gradient systems as well as quaternary low pressure gradient systems.
Two types of multilayer solvent blending reactors (Shimadzu patent) allow solvent mixing with minimized system dwell volumes. Depending on requirements, 180 µL or 20 µL reactors can be utilized.
In terms of carryover and reproducibility, the autosampler SIL-30AC exceeds the performance of any other HPLC autosampler on the market. For critical samples and matrixes, carry-over can be eliminated by the use of three rinsing solvents (plus one external needle wash solvent) in combination with various rinse modes before and after sample aspiration.


 Figure 3: Inside view of CTO-30A. First figure: Heating block and separated heating cartridges. Second figure: Low volume low/dispersion pre-heater. Third figure: Column compartment.
Figure 3: Inside view of CTO-30A. First figure: Heating block and separated heating cartridges. Second figure: Low volume low/dispersion pre-heater. Third figure: Column compartment.
In addition to the well-established and precise DIIMS technique (Direct Injection with Isolated Metering System), the autosampler also offers fixed-loop injection for ultra-fast separation with minimized peak width and gradient delay. An overlapping injection function is standard with Nexera enabling maximum reduction in cycle time.
For high-temperature LC analysis up to 150 °C, Nexera offers efficient pre-heating for solvents to enable stable and reliable conditions. The advanced Intelligent Heat Balancer (IHB) minimizes band-broadening during high-temperature analysis through use of independently controlled heat zones within the column compartment, ensuring accurate column temperature regardless of flow rate. The post-column cooler can reduce detector noise when equipped for high-temperature analysis.
Why select a system exceeding requirements?
The following example shows a series of runs to determine the content of an active ingredient. The User Requirement Specification for Peak Area Precision (injection volume = 5 µL) is ≤ 2 % RSD.
The overlay of the chromatograms (Figure 4) shows good precision, but in the zoomed view a clear trend towards decreasing peak area is apparent. The % RSD for peak area precision is within the limit of 2 % RSD, but the trend (Figure 5) indicates a problem. It is therefore possible to intervene and avoid out-of-specification results.
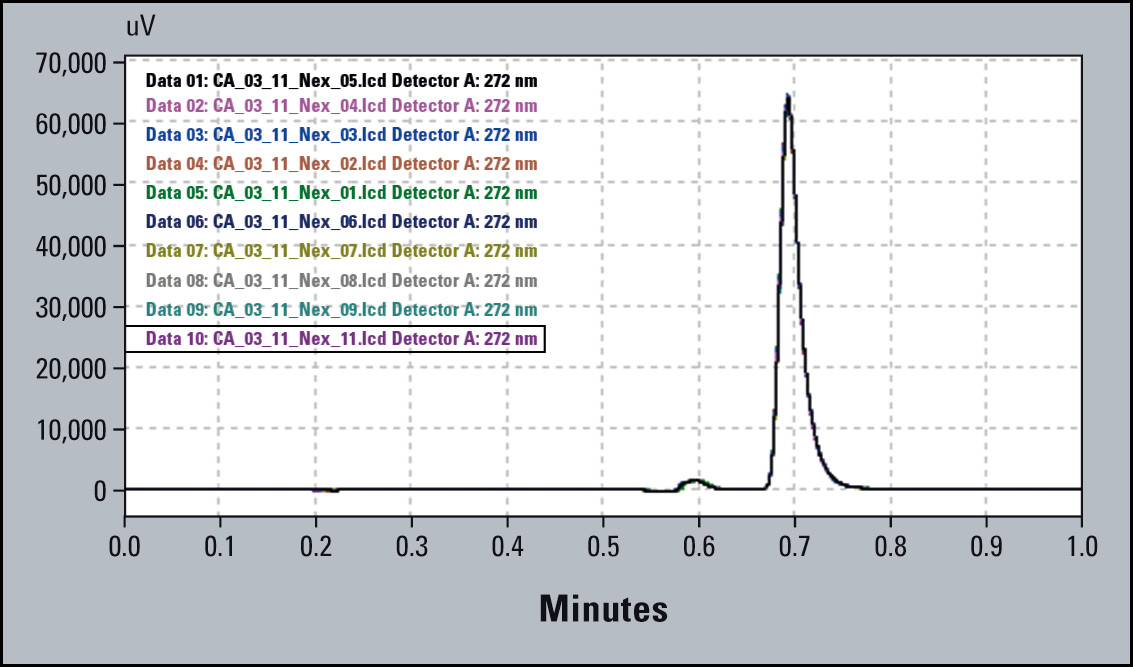
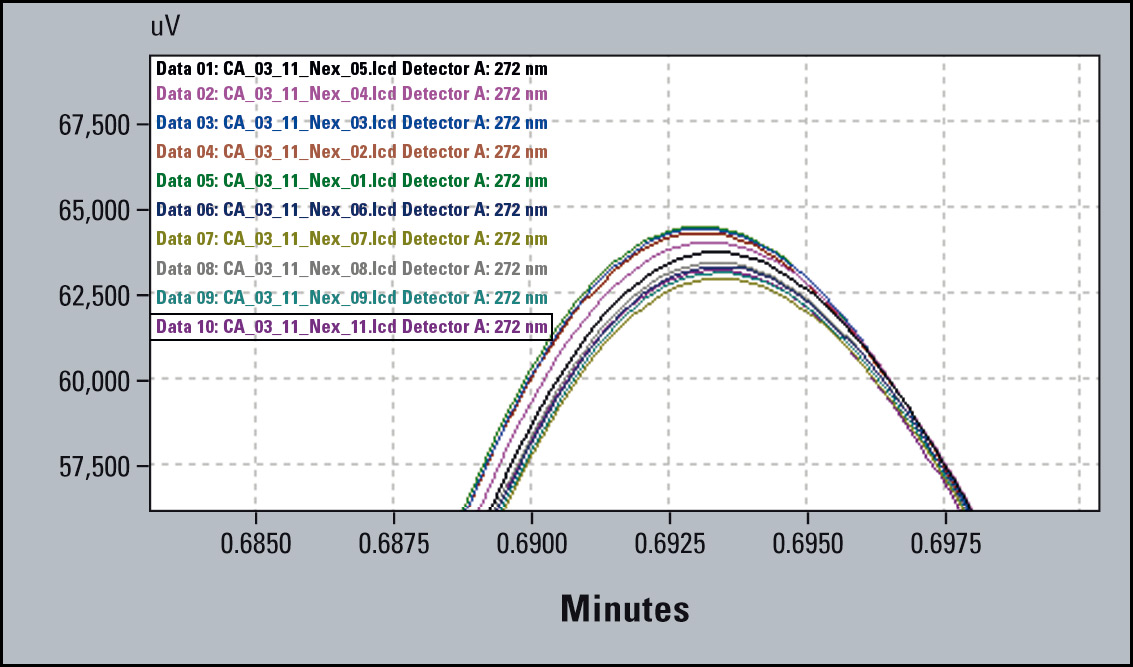 Figure 4: Chromatogram Overlay of 10 subsequent runs (Right Part: Zoomed Peaks)
Figure 4: Chromatogram Overlay of 10 subsequent runs (Right Part: Zoomed Peaks)
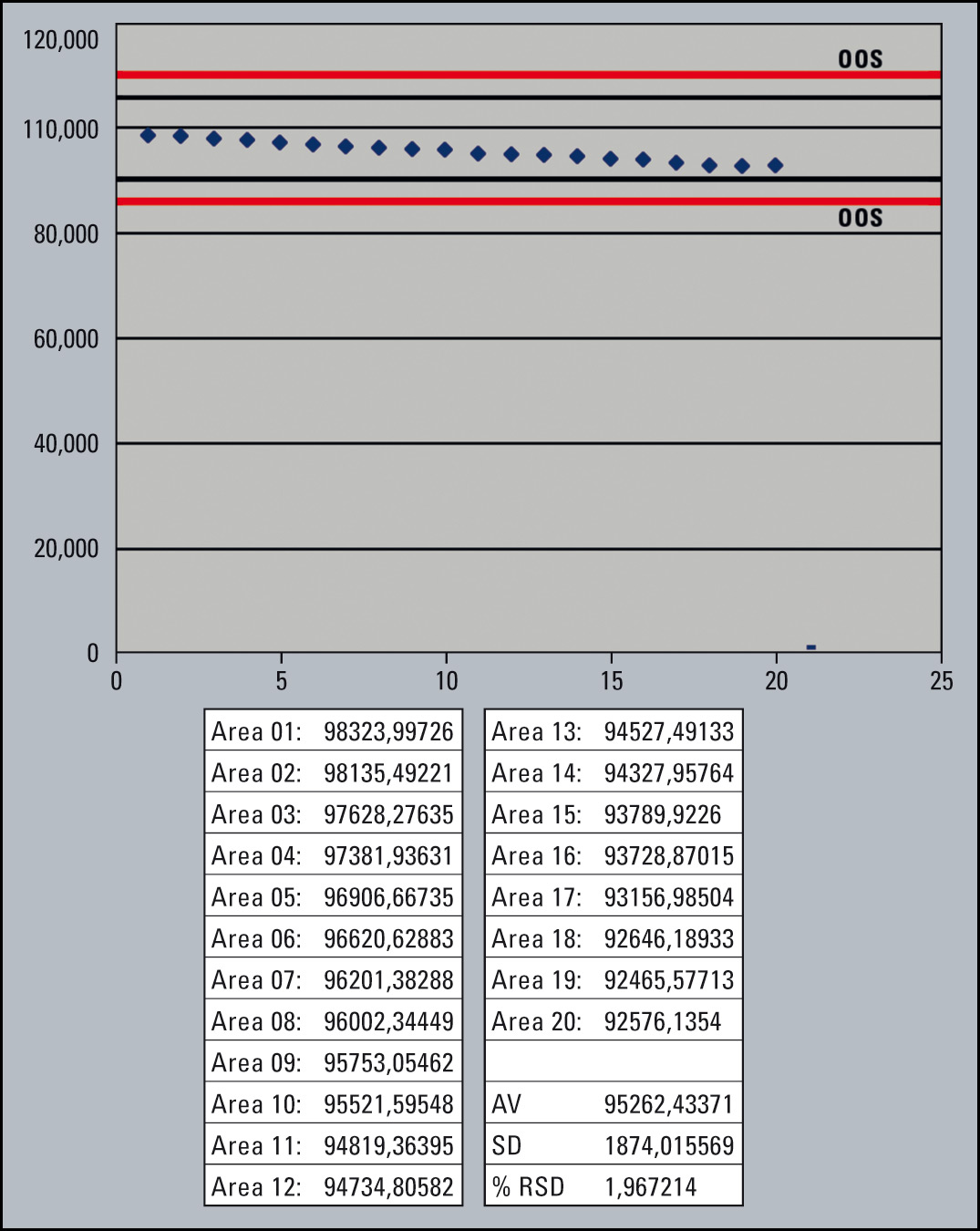 Figure 5: Trend Plot of Peak Areas
Figure 5: Trend Plot of Peak Areas
Carryover works against productivity:
Particularly in drug discovery, sample carryover may cause delays and additional costs, since false positive candidates pass through the entire process. With DIIMS (Direct Injection with Isolated Metering System) construction and well selected materials of the SIL-30AC autosampler, carryover is reduced to a minimum. By applying special rinse modes sample carryover can be reduced to an undetectable amount. The figure below shows 0.0006 % carryover for a Chlorhexidine injection.
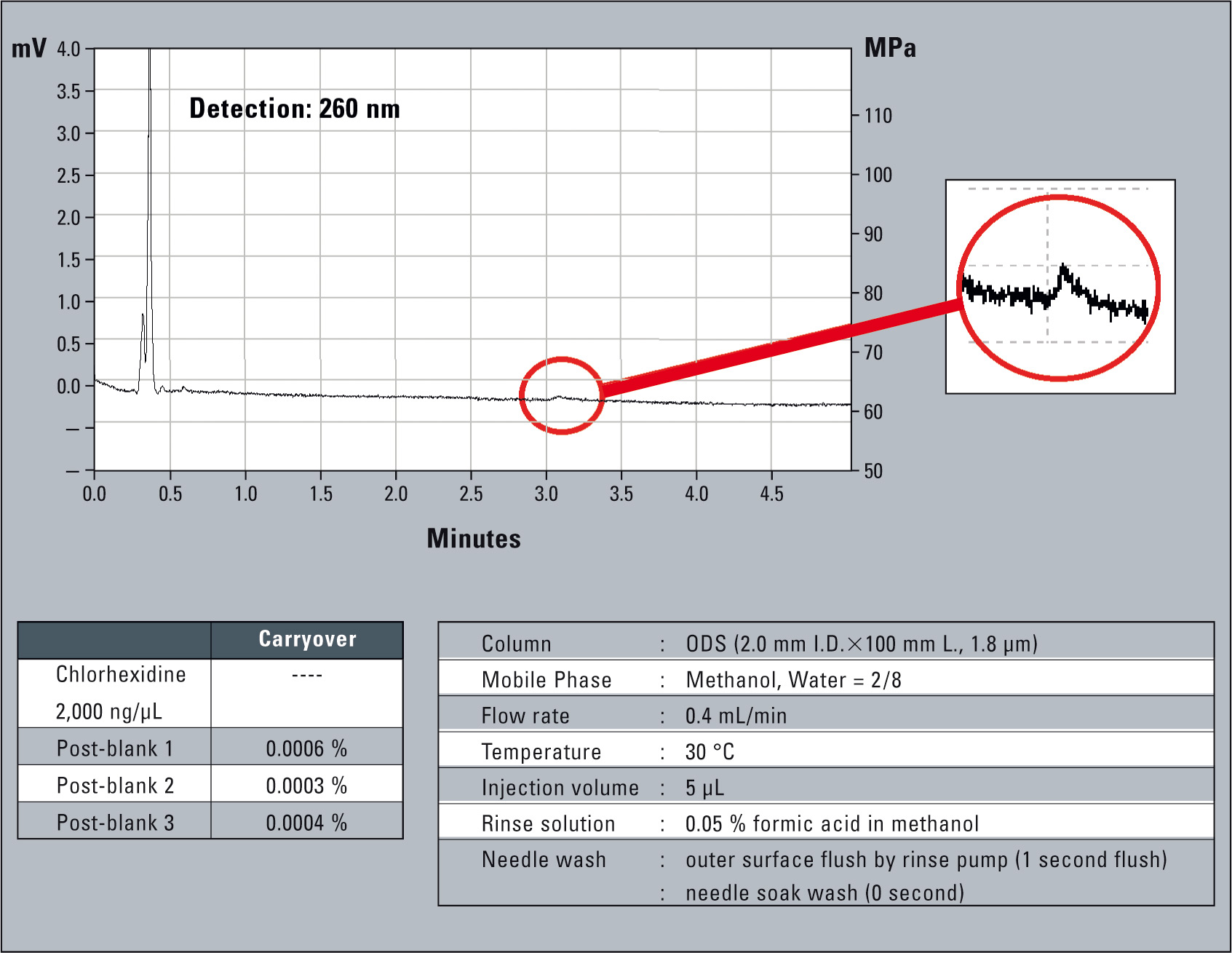 Figure 6: Carryover of chlorohexidine without injection port rinse, needle internal rinse
Figure 6: Carryover of chlorohexidine without injection port rinse, needle internal rinse
By applying additional rinsing steps, carryover can be eliminated completely as shown in figure 7.
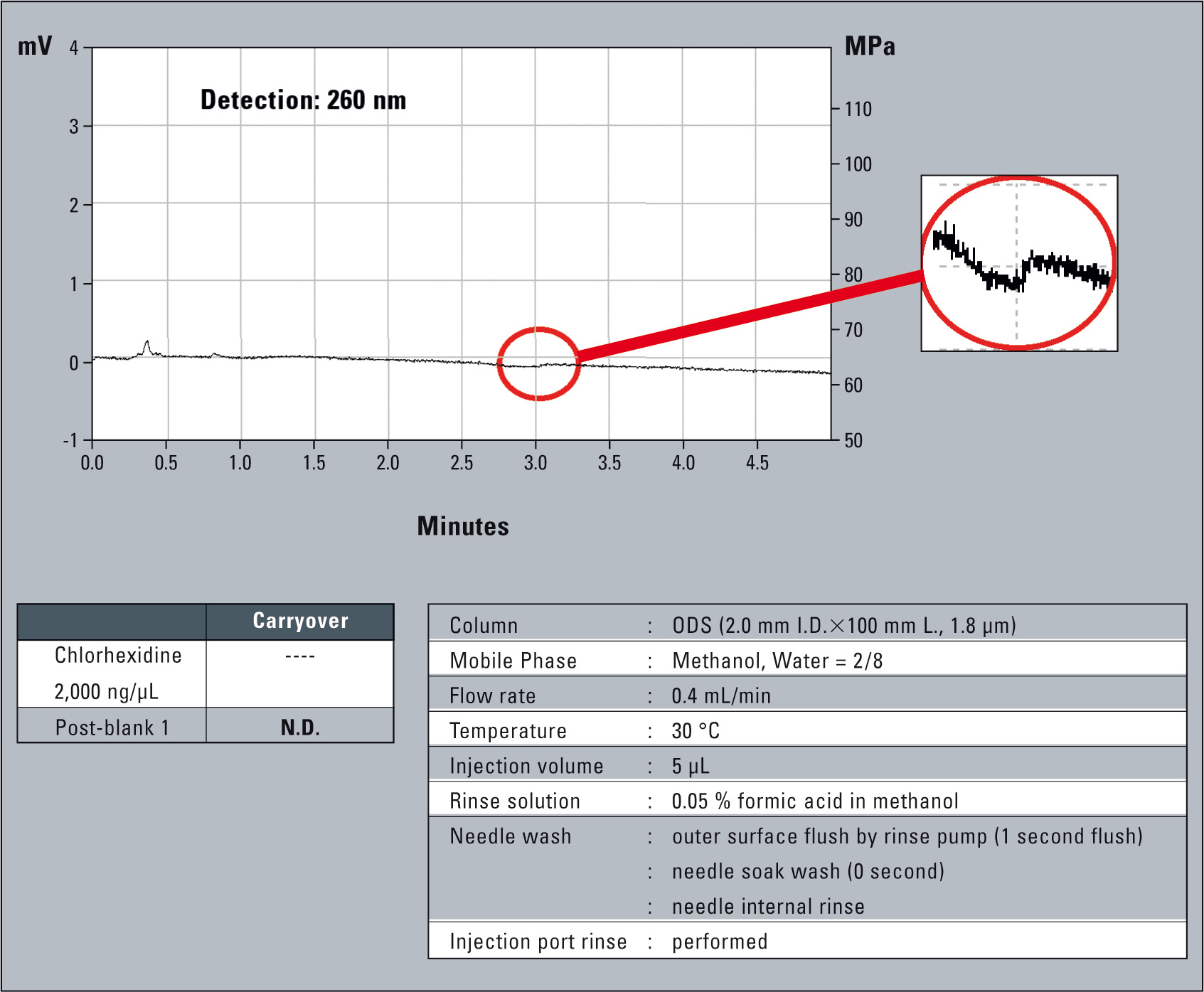 Figure 7: Carryover of chlorohexidine with injection port rinse, needle internal and outer surface rinse
Figure 7: Carryover of chlorohexidine with injection port rinse, needle internal and outer surface rinse
These are just a few examples demonstrating the advantages of a high end UHPLC system. The Nexera system provides the features and flexibility to cope with all demands expected of a modern UHPLC used in the Pharmaceutical Industry. In the final analysis, system reliability and long maintenance interval defines the real value in daily routine use. This was taken into account when designing the Nexera. The result is a reliable, robust and precise future-proof UHPLC system.