How heavy metals can migrate into pharmaceuticals
EDX series detects elements from C to U – carbon and uranium
Pharmaceutical substances can be toxic and dangerous to the human health if not manufactured with proper care. All substances from starting materials to finished products are therefore strictly regulated. In general, this covers proof of product stability, drug-release profiles and examination of the active pharmaceutical ingredients (API). Furthermore, product safety including sterility and absence of non-proprietary materials, e.g. heavy-metals is determined.
One of the analytical tests in the pharmaceutical industry is the quantification of heavy metals or inorganics in all substances and materials in a pharmaceutical product. For example, toxic heavy metals such as cadmium (Cd), mercury (Hg) and lead (Pb) are checked normally. It is also common practice to measure catalysts such as palladium (Pd) and platinum (Pt). Throughout the manufacturing processes, there are many potential sources of contamination. In addition to testing the raw materials it is therefore essential to also measure all finished products and in some cases intermediates to guarantee compliance with the appropriate regulations.
Contamination by the packaging
But even when the product is produced without contamination, the final product can contain heavy metals. Contamination can be caused by the packaging material, for example by PET bottles containing antimony (Sb) as a catalyst. Residues of antimony remain in the bottle and can, under certain conditions, migrate into the pharmaceutical product. It is therefore logical to control heavy metal content in the packaging material.
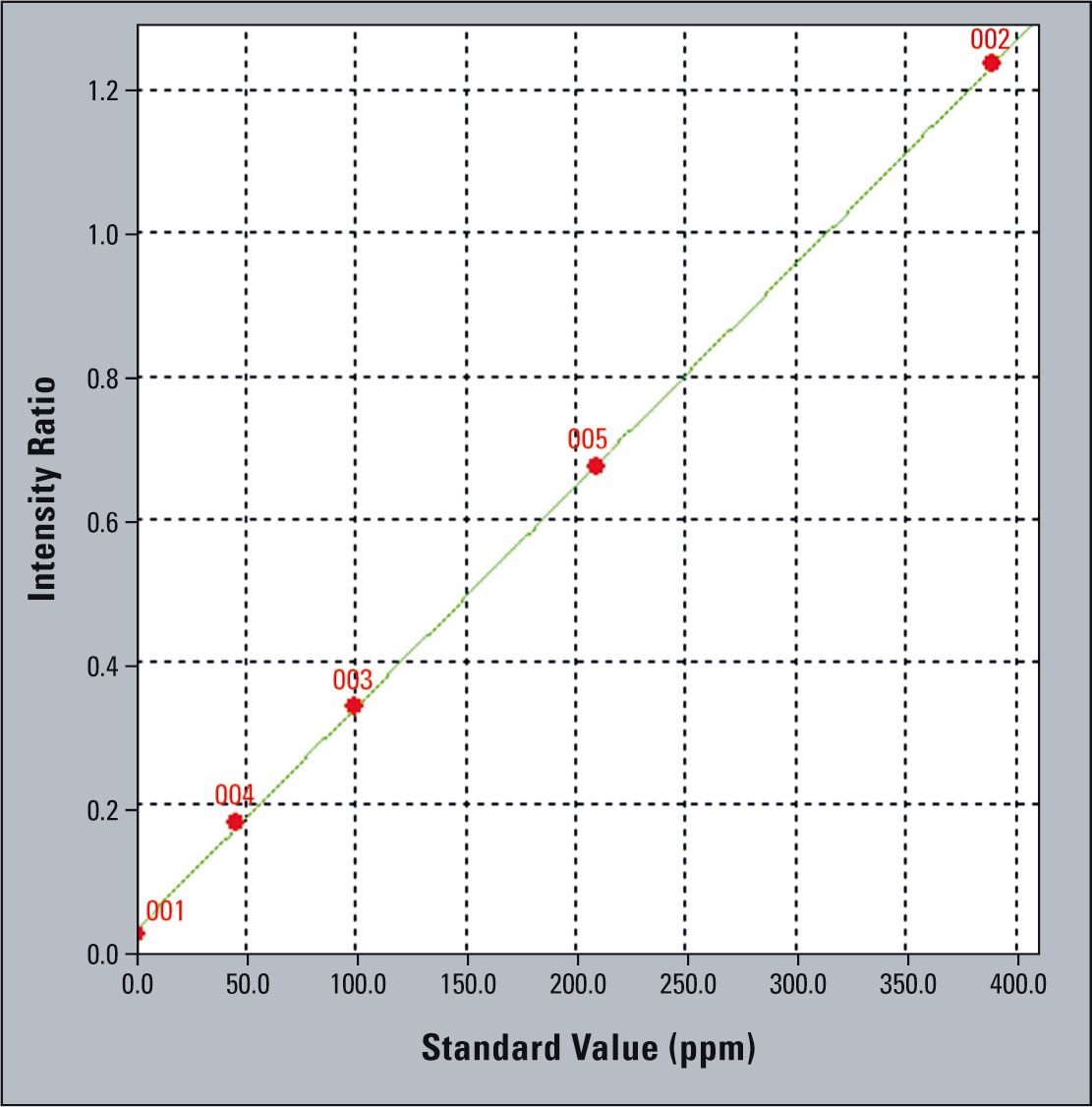 Figure 1: Measurement of a PET bottle containing around 250 ppm of Sb
Figure 1: Measurement of a PET bottle containing around 250 ppm of Sb
Is there evidence that antimony migrates into the product?
Unfortunately, yes. Scientists at the University of Copenhagen, Denmark, recently studied antimony levels in 42 juice drinks and found concentrations above EU limits for drinking water in eight of them.
Freshly produced juice drinks appear to be free from increased antimony content. The acid in the juice seems to enhance the migration process of antimony from the bottle into the liquid contained. PET bottles are used not only for juice drinks, but also for pharmaceutical products.
This discovery is of concern since antimony is a known toxic element. In small doses, it can cause headaches, dizziness and depression. Larger doses can lead to violent and frequent vomiting, and even to death within a few days.
Shimadzu’s EDX series of x-ray fluorescence spectrometers has been designed for the analysis of a wide variety of elements from carbon (C) to uranium (U), in concentrations from 100 % down to a few ppm. The instruments measure the content of heavy metals in pharmaceuticals products and their packaging materials. To demonstrate the performance of the EDX instrument a calibration was made using plastic standards ranging from 50 ppm to 400 ppm.
 Figure 2: EDX measurements are a great tool for analyzing pharmaceutical products and their packaging materials in a fast and cost-efficient way
Figure 2: EDX measurements are a great tool for analyzing pharmaceutical products and their packaging materials in a fast and cost-efficient way