Residual Solvents
U.S. Pharmacopoeia – revised General Chapter Monograph <467>
Satisfy new method requirements using the Shimadzu GC-2014 and HT200H
PResidual solvents are trace-level chemical residues in drug substances and drug products that are byproducts of manufacturing, or that form during packaging and storage. It is the drug manufacturers’ responsibility to ensure that these residues are removed, or are present only in limited concentrations.
The United States Pharmacopeia (USP) recently revised General Chapter <467> on residual solvent analysis by adopting the International Committee on Harmonization (ICH) Q3C guidelines. The new revisions also include analytical methods for the identification, control and quantification of residual solvents that are adopted from the European Pharmacopoeia (EP). This revision, effective July 1, 2008, replaces previous methods and significantly increases the requirements with which a pharmaceutical company must comply in order to demonstrate that all drug products (not just new) are compliant with Chapter <467> limits. The change increases the number of solvents requiring testing from seven to fiftynine.
While most companies have extensive data on the solvents used in the manufacturing of their API, the information regarding solvents present in the excipients is usually much lower. The drug product manufacturer is responsible for control of the limit of solvents.
Testing is only required for those solvents used in the manufacturing or purification process of drug substances, excipients or products. This allows each company to determine which solvents it uses in production and to develop testing procedures addressing their individual needs.
Solvents and health risks
Solvents have been classified into three main categories based on their potential health risks:
Class 1: Solvents should not be used due to unacceptable toxicities or deleterious environmental effects.
Class 2: Solvents should be limited because of inherent toxicities.
Class 3: Solvents may be regarded as less toxic and of lower risk to human health.
Method overview
The USP has provided a method for the identification, control and quantification of residual solvents. For solvents of Class 1 and 2, the method calls for a gas chromatograph analysis with flame ionization detection (FID) and a headspace injection from either water or organic diluent. The monograph has suggested two procedures for qualitative analysis:
Procedure A specifies a G43 (Zebron ZB-624 or equivalent) phase and Procedure B specifies a G16 (Zebron ZB-WAXplus or equivalent) phase. Procedure C is for quantitative analysis. Procedure A should be used first.
If a compound above the specified concentration limit is determined, then Procedure B should be used to confirm its identity. Since there are known co-elutions on both phases, the orthogonal selectivity ensures that co-elutions on one phase will be resolved on the other. Neither of the procedures is quantitative, so to determine the concentration, the monograph specifies Procedure C, utilizing whichever phase gives the fewest co-elutions.
Procedure A: G43 (6 %-cyanopropy -94 % dimethylpolysiloxane)
Procedure B: G16 (polyethylene glycol)
Procedure C: G43 or G16 depending on the procedure with the fewest co-elutions
Class 3 solvents may be determined by <731> Loss on Drying unless the level is expected to be > 5000 ppm or 50 mg. If the loss on drying is > 0.5 %, then a water determination should be performed using <921> Water Determination.
USP monograph <467> allows the use of alternate methodologies as long as they have been validated appropriately. However, only the results obtained by the procedures given in the general chapter are conclusive. So, the results from the alternate method will have to be compared to the monograph before being acceptable to the Food and Drug Administration (FDA).
Instrumentation
All procedures were performed using a Shimadzu GC-2010, an HT200H headspace autosampler (Figure 1) and Phenomenex GC columns.
 Figure 1: GC-2014 and HT200H
Figure 1: GC-2014 and HT200H
The Shimadzu GC-2010 offers high-end GC performance ready for all analytical tasks including full fast GC functionality with filter time constant and sampling frequency of 4 ms and 250 Hz respectively for all GC detectors.
The HT200H headspace autosampler has been specially designed to meet the needs of General Chapter <467>. The sensitivity and reproducibility have been exhaustively evaluated at multiple locations around the world to ensure consistent method. The sampler has also demonstrated performance characteristics for drug substances, excipients and drug products. All show extremely consistent data.
Procedure A – Identification (Class 1 and 2 Solvents)
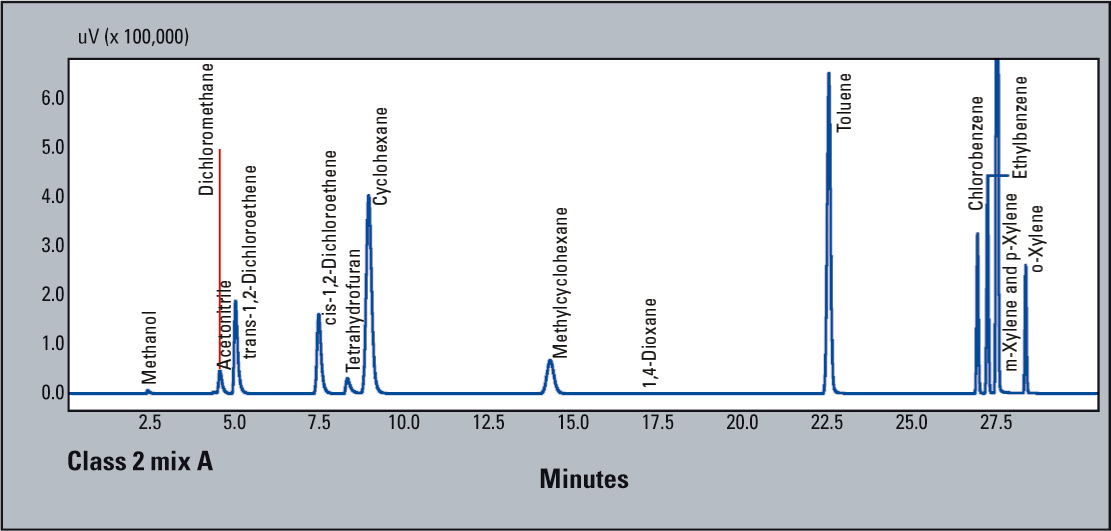 Figure 2: USP Method <467> Procedure A: Class 2 mix A for water soluble compounds
Figure 2: USP Method <467> Procedure A: Class 2 mix A for water soluble compounds
- System suitability requirements: signal-to-noise ratio of 1,1,1-trichloroethane > 5
- signal-to-noise ratio of each peak of Class 1 solvent should be > 3
- resolution between acetonitrile and methylene chloride > 1.0
- At the concentration limits specified by the monograph, signal-to-noise ratio for 1,1,1-trichloroethane was 59.9; and all other compounds exceeded 3. Resolution between acetonitrile and methylene chloride was 1.71 (Figure 2).
Conditions
Gas chromatograph: Shimadzu GC-2010
Injection: Split 5:1 @ 140 °C, 1 mL
Carrier gas: Helium @ 35 cm/s (constant linear velocity)
Oven program: 40 °C for 20 min to 240 °C @ 10 °C/min for 20 min
Detector: FID @ 250 °C
HT200H headspace: 60 minute conditioning at 80 °C, syringe at 85 °C, 0.5 min on/off shaker, 1 min flush
Column: Zebron ZB-624, 30 m x 0.32 mm x 1.8 µm
Procedure B – Confirmation (Class 2 and 3 Solvents)
- System suitability requirements: signal-to-noise ratio of benzene > 5
- signal-to-noise ratio of each peak of Class 1 solvent should be > 3
- resolution between acetonitrile and trichlorethylene chloride > 1.0
- At the concentration limits specified by the monograph, signal-to-noise ratio for benzene was 104.2; and all other compounds exceeded 3. Resolution between acetonitrile and trichloroethylene chloride was 1.52 (Figure 3).
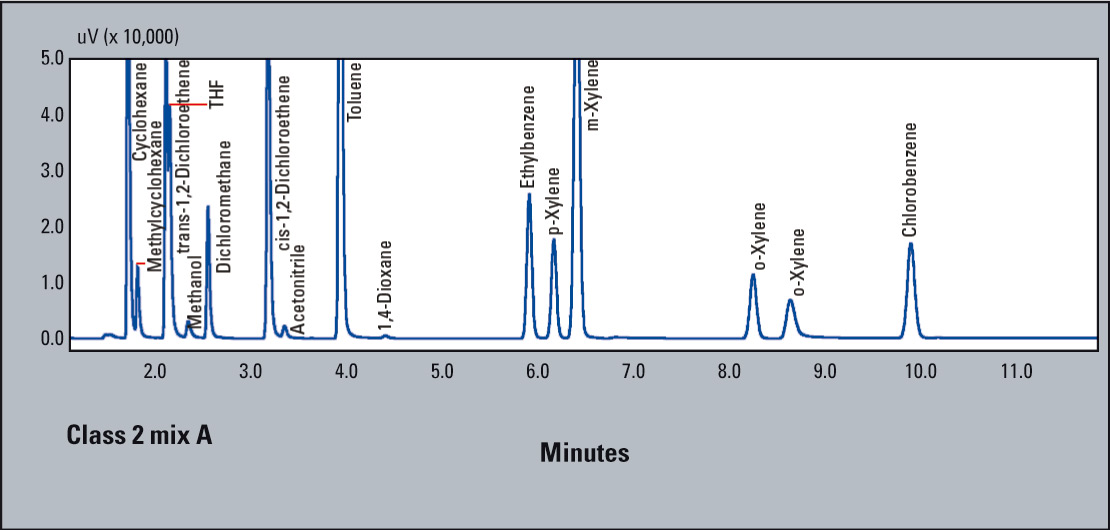 Figure 3: USP Method <467> Procedure B: Class 2 mix A for water soluble compounds
Figure 3: USP Method <467> Procedure B: Class 2 mix A for water soluble compounds
Conditions
Gas chromatograph: Shimadzu GC-2010
Injection: Split 5:1 @ 140 °C, 1 mL
Carrier gas: Helium @ 35 cm/s (constant linear velocity)
Oven program: 50 °C hold 20 min to 165 °C @ 6 °C/min hold 20 min
Detector: FID @ 250 °C HT200H headspace: 60 minute conditioning at 80 °C, syringe at 85 °C, 0.5 min on/off shaker, 1 min flush
Column: Zebron ZB-WAXplus, 30 m x 0.32 mm x 0.25 µm
Conditions
All pharmaceutical companies need to determine as soon as possible how the changes to General Chapter <467> will impact their testing procedures. The more excipients and excipient vendors a company uses, the more effort it will require to demonstrate compliance with the new methodology.
The new USP regulations are aimed at improving patient safety and will need to be implemented for all products, whether existing or new. Although the USP has provided a testing method that can be used to identify and quantitate most Class 1 and 2 solvents, the method can be improved based on each companies needs. However, if a company changes any of the procedures specified by <467>, the new method will need to be fully validated. Only those solvents used in the manufacturing process must be tested in the final dosage form.
For the best solution, each company must consider the number of samples, analysis time, method validation, accuracy, precision and cost of equipment. Once method performance has been achieved, it is also important to consider whether the method can be transferred to other manufacturing facilities. Do they have the knowledge and instrumentation to implement the method?
The changes to the <467> monograph have applied since July 1, 2008, and it is essential to formulate a strategy now for compliance. During the process, many questions and concerns will undoubtedly arise. To ensure the USP addresses as many of these concerns as possible in the new method, an open dialog between industry and the USP is criticial.
The combination of a Shimadzu GC-2010 with an HT200H headspace autosampler is a cost-effective solution to meet the needs of static headspace injection with GC analysis.