High-precision analysis of mineral waters
Atomic absorption spectroscopy – Microsampling method analyzing Na, K, Ca and Mg
 Source: Werner Krug / www.gourmetreise.at
Source: Werner Krug / www.gourmetreise.at
Consumption of bottled water has been increasing steadily in the last years. The average consumption of mineral water was approximately 140 liters per person in Germany in 2010. The trend is still increasing, since bottled water is a healthy and inexpensive drink in comparison to other soft drinks.
Mineral waters contain minerals and other dissolved substances which influence the taste or add therapeutic value. Mineral waters are generally obtained from naturally occurring mineral springs or sources. The dissolved substances in either sparkling or still water consist of various salts, carbonate and sulfur compounds.
The German test organizations “Öko-Test” and “Stiftung Warentest” monitor the quality of German and European mineral waters. In 2010, they have evaluated more than 100 mineral waters and found some important contaminations with heavy metals such as arsenic, boron and manganese.
Furthermore, the concentration of alkaline and alkaline earth elements did not match the certified values on the labels. For example, mineral water which is declared as calcium-rich must have a minimum concentration of 150 mg/L calcium. On the other hand, a low-sodium water must not exceed a concentration of 20 mg/L, as this type of water is often used for therapeutic purposes.
The concentration levels of the essential and toxic elements have to be controlled so that a constant water quality can be maintained. Quantitative measurements of elements at the trace and ultra-trace concentration levels are carried out using atomic absorption spectrophotometers such as the Shimadzu AA-7000 in flame and graphite furnace atomization (Figure 1).
 Figure 1: AA-7000
Figure 1: AA-7000
Flame micro sampling method vs. flame continuous method
Furthermore, the AA-7000 in combination with the ASC-7000 sample preparation station allows the automated flame micro sampling method (Figure 2). In this method, the flame atomic absorption analysis is conducted with small sample volumes (2 – 90 µL), while in the conventional flame method (hereafter “flame continuous method”), the sample is continuously aspirated with a flowrate of approximately 8 mL/ min and larger sample volumes are needed for aspiration.
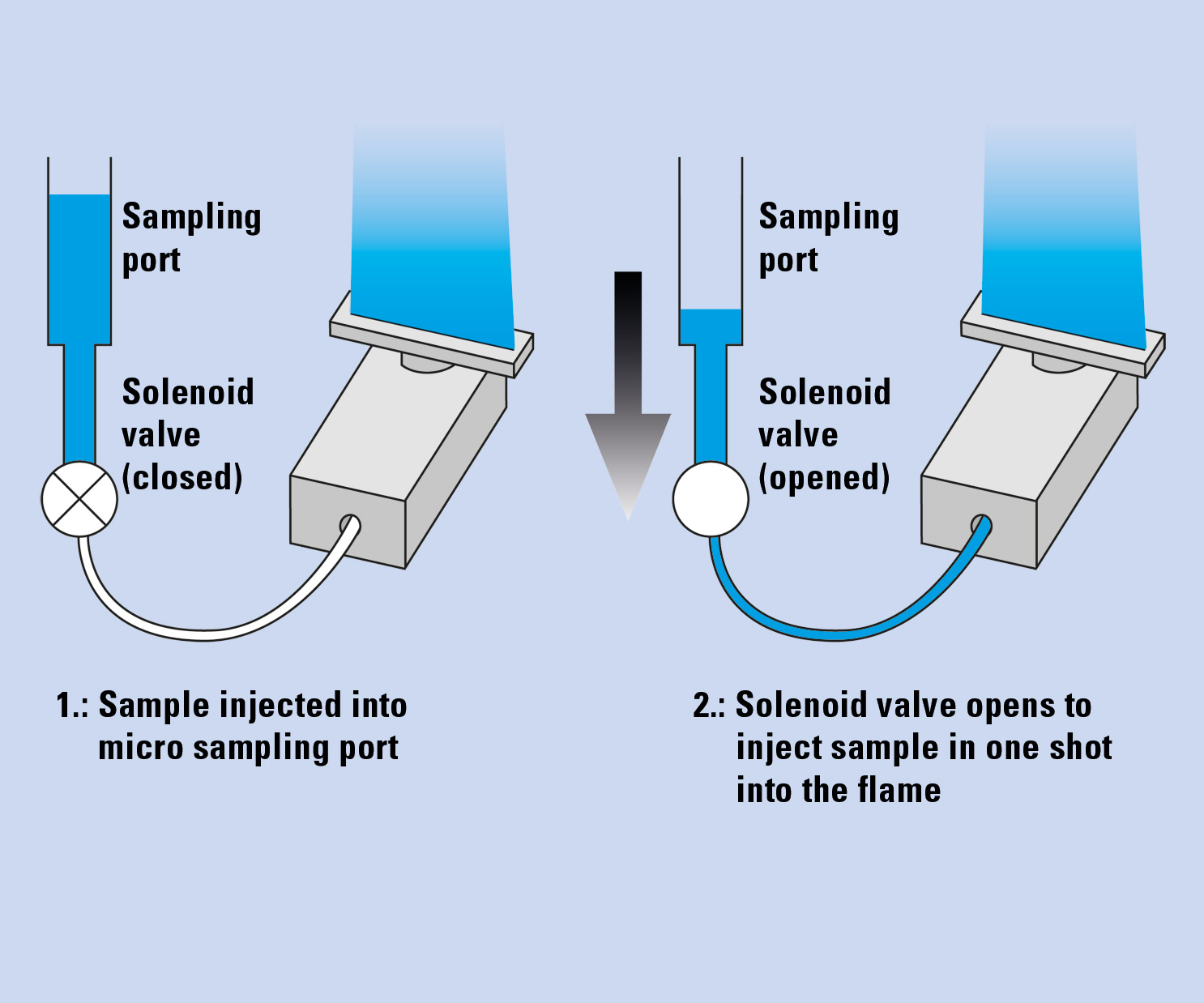 Figure 2: Microsampling method
Figure 2: Microsampling method
The flame micro sampling method has several advantages over the flame continuous method: analysis is possible with a small amount of sample, and when the autosampler is used, automatic dilution of the sample and automatic addition of buffer solutions are possible in order to compensate for interferences.
Moreover, since only a small amount of sample is introduced, the flame micro sampling method is effective for analysis of high matrix samples which may cause clogging of the burner in the flame continuous method. So the method is the right choice for determination of alkaline and alkaline earth elements in mineral water.
Sodium, Potassium, Calcium and Magnesium belong to the essential mineral substances in the human organism. These elements influence the generation of enzymes and hormones, control the osmotic pressure in tissues and body fluids and are important for the exchange procedures in the cell membranes. The recommended daily amounts (Na: 550, K: 2,000, Ca: 800 – 1,000, Mg: 350 mg/L) can be partly covered by consumption of mineral waters. But the composition of mineral waters according to the essential elements has a wide variety, and depending on the origin the composition might be quite different.
DIN/EN regulations control Na, K, Ca and Mg contents
Control of Na, K, Ca and Mg in a variety of mineral waters has been performed according to current DIN/EN regulations with the Shimadzu AA-7000 atomic absorption spectrophotometer in a fully automatic multi-element sequence. The blank, standards and the water samples are all placed in the autosampler and then mixed automatically with the corresponding reagents which have to be added according to the DIN/EN method. In the case of Sodium and Potassium, 40 µL of CsCl solution (12.65 g CsCl + 50 mL HCl (d = 1.16) filled up to 500 mL volume with H2O) will be added for a 400 µL mixing volume of standard and sample solution which is homogenized before injection to the flame. In the case of Calcium and Magnesium, a La2O3 solution (5.875 g La2O3 + 50 mL HCl (d = 1.12) filled up to 250 mL volume with H2O ) has been used.
All instrumental parameters and measuring conditions are loaded automatically from the software and combined in a multi-element sequence. These conditions are set automatically for each element including optimized burner height and gas flow rates.
Under these conditions, a series of more than 20 drinking water and mineral water samples has been analyzed. A reference material (NIST SRM 1640) was also measured as a laboratory control sample, showing an excellent recovery rate of 99 %.
AA-7000 in combination with the microsampling kit is a “state of the art” atomic absorption spectrophotometer for high precision measurements of element concentrations in mineral waters.