Mercury in your eye
X-ray fluorescence analysis – Hg content in contact lens solutions
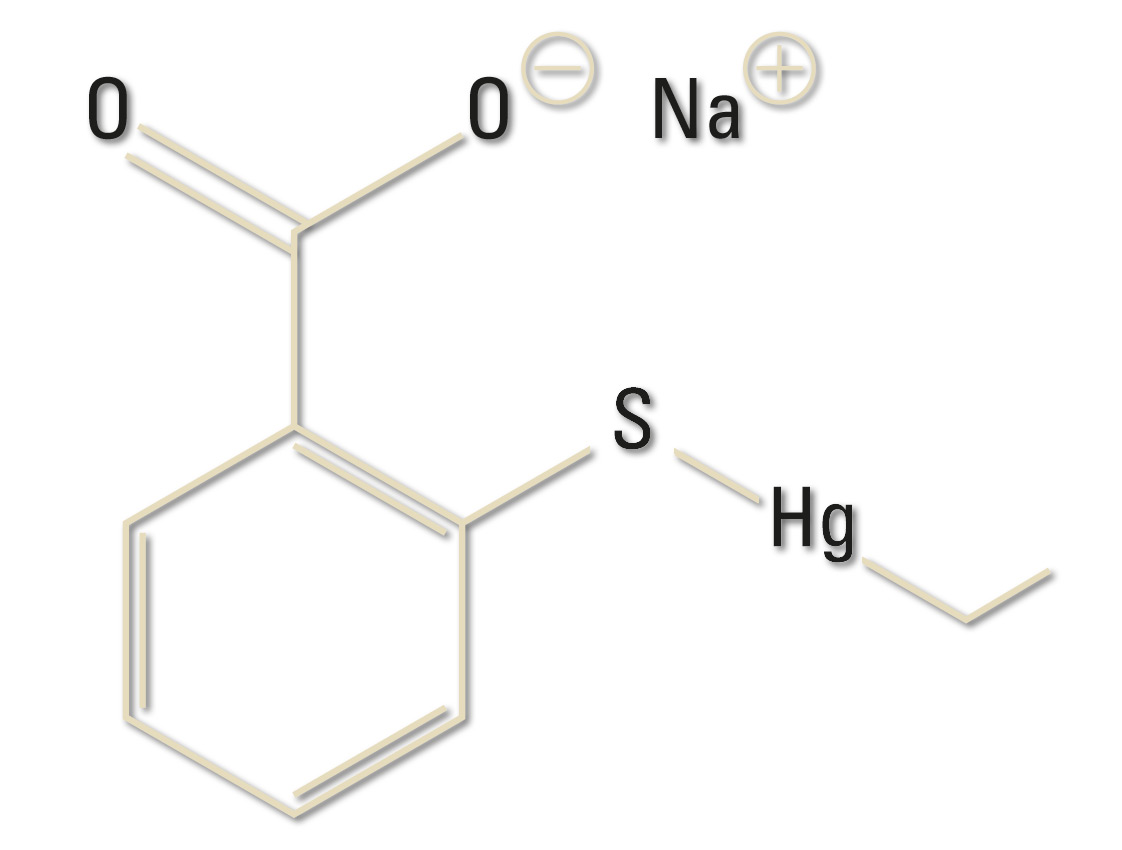
Mercury has been known for ages, and can be found in ancient Greek history. In the middle ages it was used for medical treatments, and very often it was over-dosed. Today, the element is also part of everyday products such as sterile solutions for cleaning contact lenses. One of the components of the lens solution may be Thiomersal (commonly known as Thimerosal in the US).
Thiomersal is a mercury-based preservative used since the 1930s in the manufacture of vaccines and other pharmaceutical products such as antiseptics, and also for cosmetics and contact lens solutions. It is used to prevent bacterial and fungal growth. During vaccine production it has also been used both to inactivate certain organisms and toxins and to maintain a sterile production line.
Mercury and mercury compounds
Mercury is a naturally occurring element found in the earth’s crust, soil, water and the air. It is released into the environment by volcanic eruptions, weathering of rocks and the burning of coal. Through the food chain mercury can find its way into the human body.
Mercury is toxic independent of its forms
The effects of exposure to mercury on health depend on its chemical form (elemental, inorganic or organic), the route of exposure (inhalation, ingestion or skin contact) and the level of exposure. Depending on the type and amount, exposures to mercury can damage the nervous system, kidneys, liver and immune system.
The two organic forms of mercury, methylmercury (found in fish) and ethylmercury (in thiomersal), are closely related but have important differences. Methylmercury is more potent. Accumulation in the body is more likely since the time needed for the body to eliminate it (known as the half life) is about 50 days. Ethylmercury does not accumulate in the body to this extent since its half life is only about 7 – 10 days.
Ethylmercury is rapidly converted in the body to inorganic mercury and excreted. Mercury becomes harmful when it reaches a certain level in the body. The toxicity depends on the amount of mercury consumed in relation to body weight. Infants are at greater risk than adults due to their lower weight. Mercury is accumulated over time.
It’s advisable to reduce total exposure to mercury in babies and young children in a world where other environmental sources (particularly in food such as fish) may be more difficult to eliminate.
A sugar cube in a bathtub
How much mercury is in a contact lens solution? It varies from country to country – in Germany up to 0.007 % of thiomersal is allowed in cosmetic products (eye make-up and eye make-up remover [Directive 76/768/EE]). In a tested contact lens solution the concentration of Thiomersal was 0.001 %. This is equal to 10 ppm or a sugar cube solved in a big bathtub (270 L).
How much mercury can be found in fish?
Predatory fish such as sharks and tuna contain rather high concentrations of mercury. A concentration of 0.5 ppm for Tuna and up to 1 ppm for sharks is quite common. Whether eating a tuna steak (200 g) for dinner or applying a drop of lens solution (0,05 mL) to your eye every morning over a period of three months – in both cases almost the same amount of mercury compound is provided to the body. Of course it’s not the same compound, and is applied differently.
X-ray fluorescence analysis measures Hg content in contact lens solutions
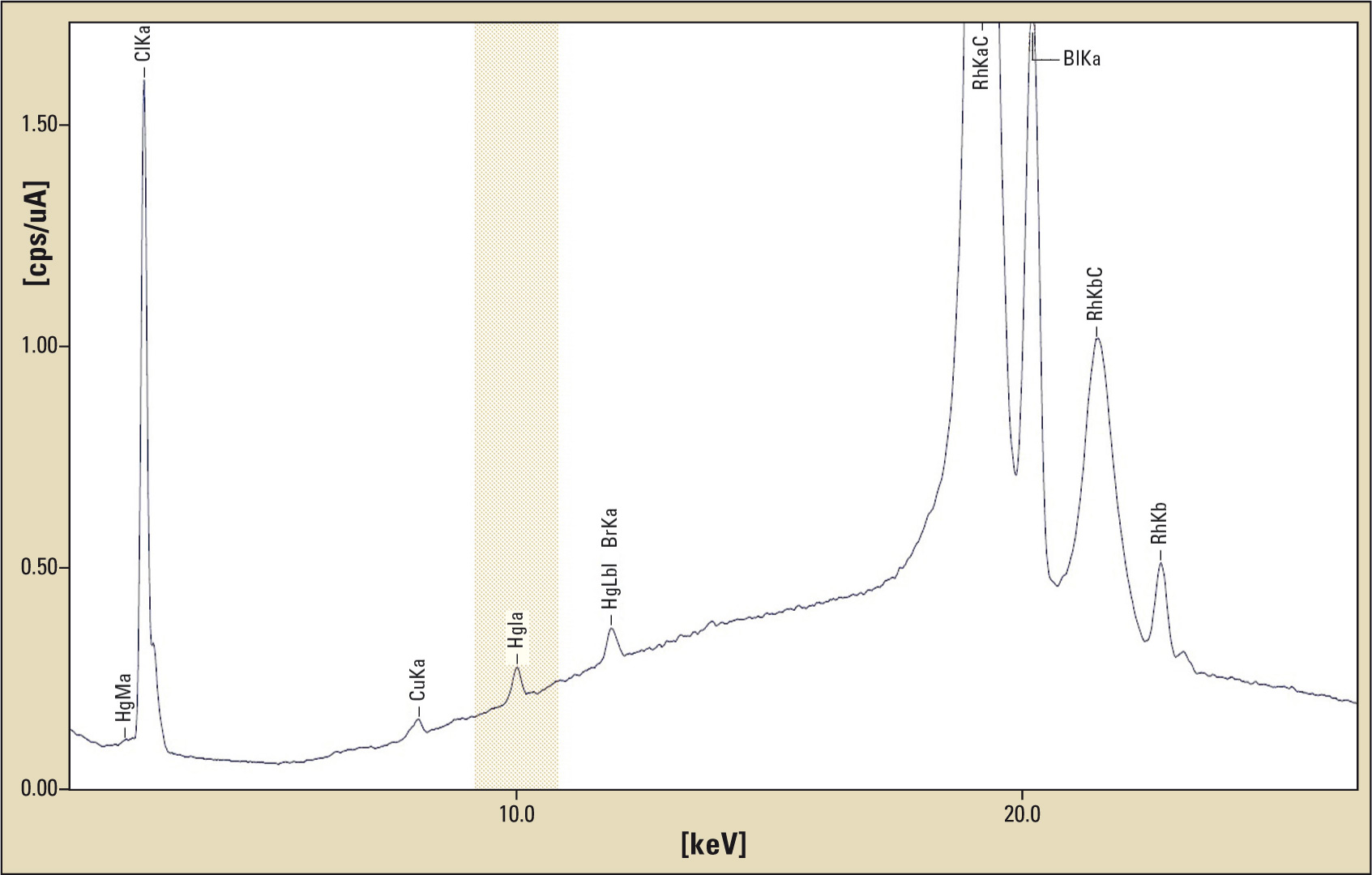 Figure 1: FP Measurement of contact lens solution measuring method: FP (fundamental parameter), measuring time: 300 s, measured Hg concentration: 41 ppm
Figure 1: FP Measurement of contact lens solution measuring method: FP (fundamental parameter), measuring time: 300 s, measured Hg concentration: 41 ppm
For analyzing lens care solutions FP-measurements (fundamental parameters) were carried out using Shimadzu’s EDX-720 X-ray fluorescence spectrometer. In order to obtain a better signal to noise ratio the sample solution was concentrated by evaporation of water to 1/10 of the initial quantity. Since nearly half of the weight of Thiomersal is due to Mercury, the expected concentration of the original sample solution is 5 ppm, and 50 ppm after evaporation of the water.
Results of the analysis met these expectations. A mercury concentration of 41 ppm (expected 50 ppm) was measured. X-ray fluorescence analysis is therefore a suitable method for determination of the concentration of mercury in fluids. It is easy-to-use, precise and reliable.