How salt load doesn’t become a heavy load
TOC determination in highly polluted samples
 TOC-L with autosampler
TOC-L with autosampler
TOC is an accepted parameter for the determination of organic pollution in various matrices. Originally used as an indicator for testing drinking and wastewater, TOC is now increasingly being used as a monitoring parameter in many processes. In the chemical industry it is, for instance, used for chlorine-alkali electrolysis brines with a salt content (NaCl) of up to 28 %. In these processes it is important to know the TOC content. The unique feature of the application does not inherently lie in the conversion of carbon compounds to carbon dioxide, but in the salt load associated with the matrix.
Are high salt loads a problem?
Thermal catalytic combustion of the sample is accompanied by crystallization of the dissolved salts. Depending on the salt load level, this can contaminate the catalyst or clog the system, requiring maintenance measures (for instance, replacement of the catalyst) to return the instrument to operational status. Of course, it is desirable to keep maintenance intervals as long as possible. Shimadzu’s TOC-L offers several possibilities to minimize maintenance frequency for highly polluted samples.
The TOC-L series features catalytic combustion at 680 °C. This is below the melting point of sodium chloride, preventing a melt from deactivating the active centers of the catalyst. The use of a platinum catalyst ensures complete conversion of the organic carbon compounds to CO2.
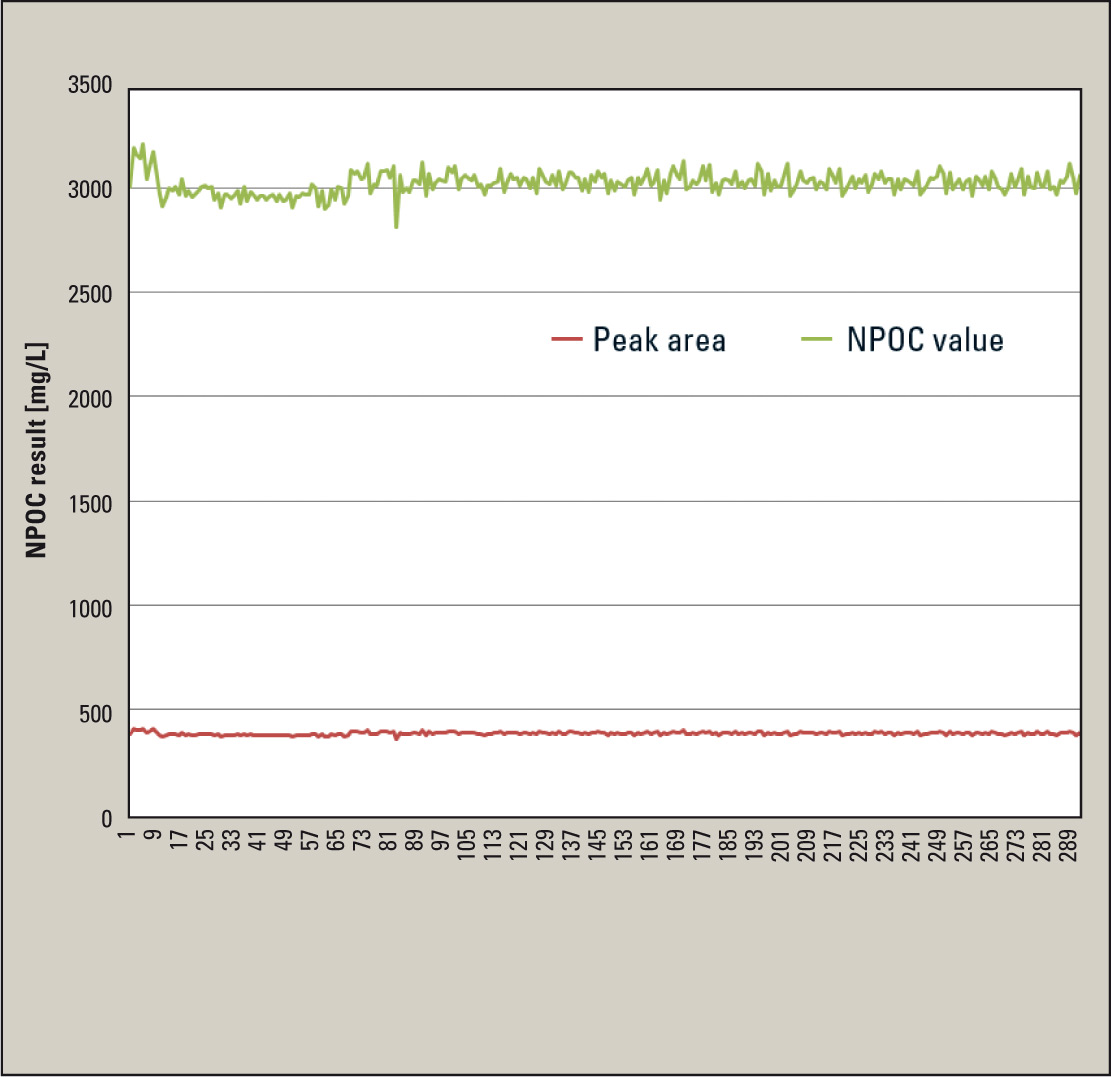 Figure 2: Determination of a wastewater using the automated dilution function (10-fold)
Figure 2: Determination of a wastewater using the automated dilution function (10-fold)
The highly sensitive NDIR detector enables small injection volumes (typically 20 – 50 µL), which minimizes the absolute sample load on the catalyst. An additional reduction can be attained using the integrated dilution function. The ISP (Integrated Sample Pretreatment) module enables automated sample dilution, which can be applied continually or when measuring values are being exceeded. In this case, the user defines the desired dilution factor in the method. Figure 2 shows close to 300 injections of a wastewater which was measured after 10-fold dilution. The result of this measurement is approximately 3024 ± 51.1 mg/L (RST = 1.7 %).
Kit for high salt loads
The TOC concentration does not always allow dilution. In such cases, the TOC-L series features a special kit for high salt loads. This kit was originally developed for the Online-TOC-4110. Based on excellent experience with this system, the salt kit was also adopted for the TOC-L series. It consists of a combustion tube with a special geometry and a special catalyst mixture. This combination prevents crystallizations that could clog the system. In this way, considerably more measurements are possible before maintenance is necessary. Sample acidification (for the NPC method) is carried out with sulfuric acid, which is used to modify the sample matrix. While NaCl has a melting point of 801 °C, the melting point of Na2SO4 is higher (881 °C). This has a positive effect on the lifetime of the combustion tube.
TOC determination of a brine
First, a calibration curve using a KHP standard solution and the automated dilution factor was created. The concentration of the standard solution was 10 mg/L. Ten points at equidistant intervals were defined in the software, which are to be derived from this standard solution. The instrument automatically does the rest (Figure 3). The respective injection volume was 50 µL.
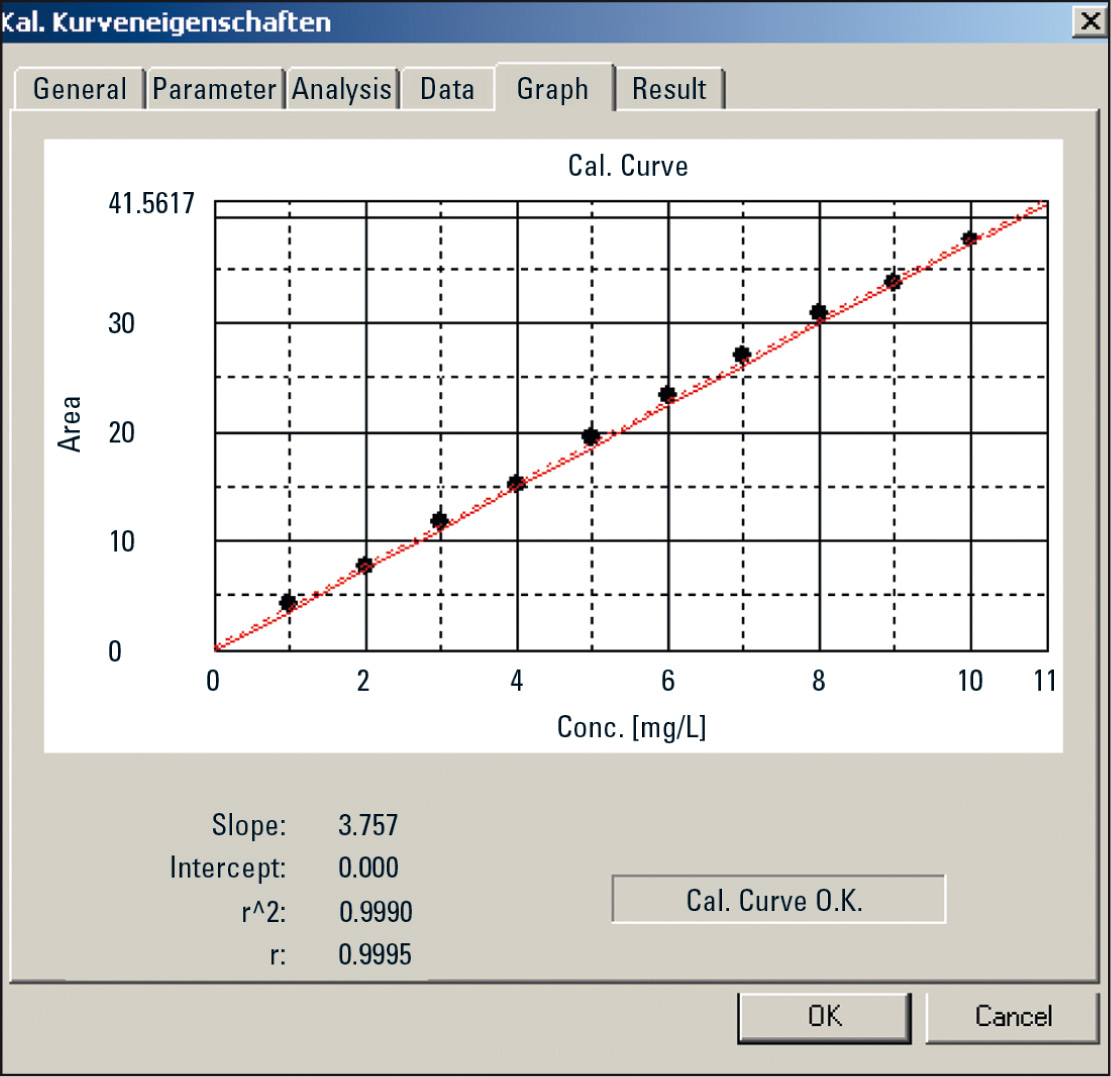 Figure 3: Calibration using the automated dilution function
Figure 3: Calibration using the automated dilution function
A 28 % NaCl solution was subsequently prepared. This was spiked with a KHP solution to a 5 mg/L TOC solution and a 15 % sulfuric acid solution was added. Initially, a blank value and a control standard (10 mg/L) were measured. Subsequently, the NaCl solution was injected. Testing of the control standards was performed after 110 and 220 injections respectively. Maintenance of the combustion tube and catalyst was not necessary after completing the measurements. Only the TOC slider block needed to be cleaned. Figure 1 shows the excellent reproducibilities and stability of the measurements.
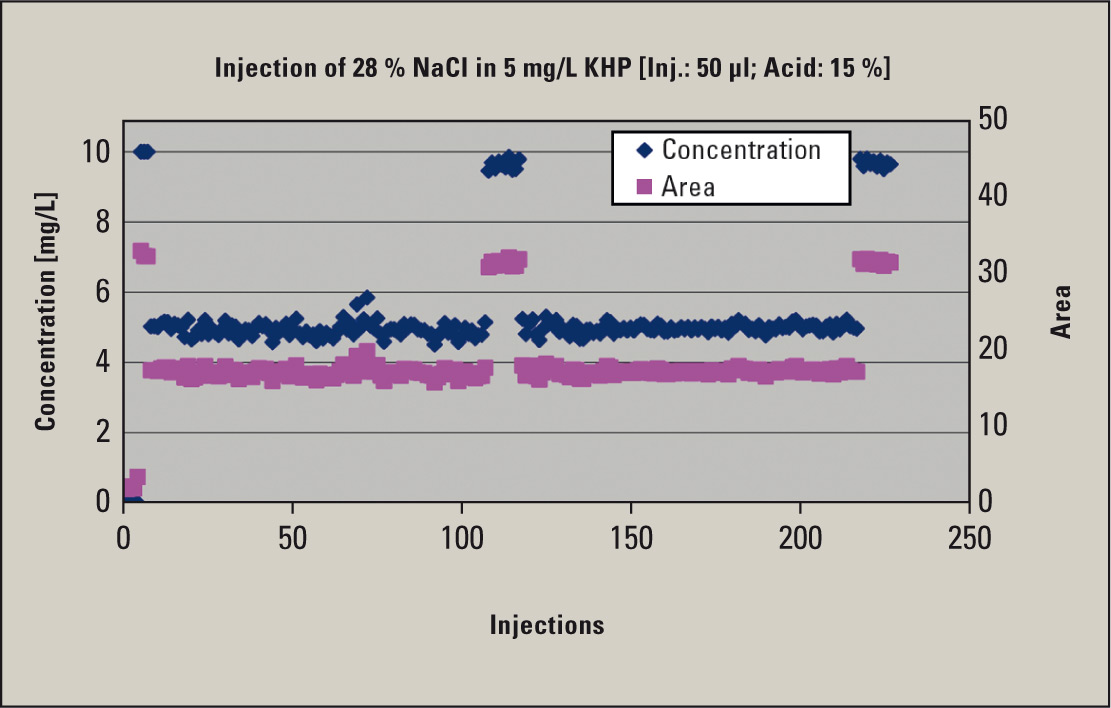 Figure 1: TOC determination of a brine (28 % NaCl)
Figure 1: TOC determination of a brine (28 % NaCl)