Burning high-tech
Graphite tubes for electrothermal atomization
 Figure 1: AA-7000 atomic absorption spectrometer with GFA-7000 graphite furnace atomizer
Figure 1: AA-7000 atomic absorption spectrometer with GFA-7000 graphite furnace atomizer
In graphite furnace technology, it is important to select the graphite tube according to the target element and sample composition. In fact, a wide variety of graphite tubes are used in electrothermal atomization. The AA-7000 atomic absorption spectrometer in combination with the high-sensitivity digitally controlled GFA-7000 graphite furnace atomizer can be operated with the following tube types: high-density graphite tube, pyrolytic-coated graphite tube, “fork”-platform graphite tube and omega platform tube®.
Made of ordinary graphite, the high-density tube is widely used. Since the hexagonal graphite crystal structure has porous characteristics, the injected sample solution permeates into the graphite tube wall during the heating process. On the other hand, the pyrolytic-coating tube has a metallic shining surface, made by formation of a pyrolytic layer through chemical vapor deposition. Since the density of the surface is higher than that of a high density tube, sample permeation into the wall is lower and the generation of the atomic cloud during the atomization stage is improved. The platform tube refers to a graphite tube in which a plate with a basin for the sample (platform) is mounted. The Shimadzu platform tube has a platform made of 100 % pyrolytic carbon. Figure 2 shows a comparison of peak profiles of pyrolytic-coated tube and the high-density tube.
High-density tube specializes in elements with low atomization temperatures
The high-density tube is used for measurements of various elements, especially for those showing low atomization temperatures, such as cadmium (Cd), lead (Pb), sodium (Na), potassium (K), zinc (Zn) and magnesium (Mg). It is also useful to reduce the measurement sensitivity of an element in case of high concentration samples.
For example, in case of aluminum, iron and copper, although a level of 1 ppb can be measured using the pyrolytic-coated tube, the sensitivity can be reduced by a factor of 100 to a level of 100 ppb using the high-density tube.
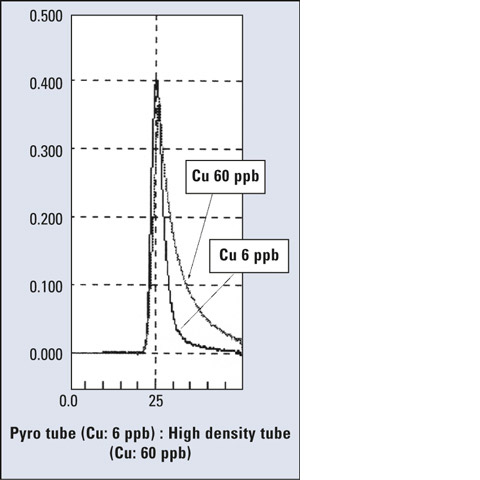 Figure 2: Comparison of peak profiles of pyrolytic-coated (6 ppb) and high density tube (60 ppb)
Figure 2: Comparison of peak profiles of pyrolytic-coated (6 ppb) and high density tube (60 ppb)
Figure 3 shows an example of calibration curves for Cu measurements using the high-density tube and the pyrolytic-coated tube. The measurement points are 20, 40 and 60 ppb in the case of high-density tube and 2, 4 and 6 ppb in the case of pyrolytic-coated tube, although similar absorbance values are shown.
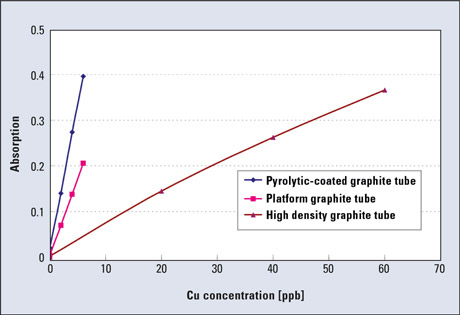 Figure 3: Sensitivity comparison of graphite tube types
Figure 3: Sensitivity comparison of graphite tube types
Pyrolytic-coated tube shows sharp peaks for carbide-forming elements
In general, the pyrolytic-coated graphite tube is the most effective solution for elements which easily form carbides as a reaction with the graphite in an uncoated tube. Nickel (Ni), calcium (Ca), titanium (Ti), silicon (Si), vanadium (V) and molybdenum (Mo) are elements typically showing this effect. In the high-density tube, a sample easily permeates into the graphite, resulting in a larger contact area between the element and carbon. In the pyrolytic-coated tube, however, a smaller contact area can suppress carbide formation, and a higher sensitivity is obtained as a result. When the peak profiles are compared, a sharp peak is observed in the case of pyrolytic-coated tube, while a broad peak is observed in the case of high-density tube. Figure 2 shows the example for copper.
Acid concentration affects sensitivity and reproducibility
Furthermore, variation of the acid concentration has a significant effect on the sensitivity and reproducibility of the analytical result. The effect of nitric acid concentration on the sensitivity of lead is shown in figure 4. When comparing different graphite tubes, the sensitivity varies more drastically as the acid concentration changes. In the case of a pyrolytic-coated tube, however, the analytical result is easily affected by the acid concentration in the sample. Compared to platform or high-density tubes, the sensitivity varies more drastically as the acid concentration changes.
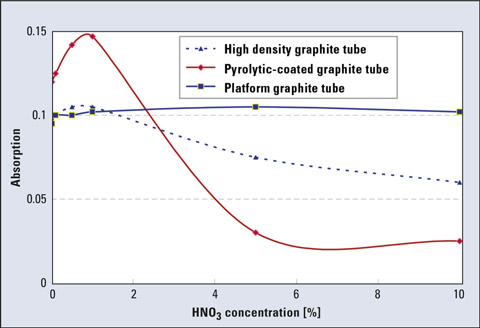 Figure 4: Effect of nitric acid concentration on Pb sensitivity among graphite tube types (Pb: 5 ppb, 10 µL injection)
Figure 4: Effect of nitric acid concentration on Pb sensitivity among graphite tube types (Pb: 5 ppb, 10 µL injection)
As a feature of the platform tube (Figure 5), the sample solution is injected onto the platform and has no direct contact with the wall of the graphite tube. It is then heated according to the element-specific heating program.
 Figure 5: Omega platform tube®
Figure 5: Omega platform tube®
During electrothermal atomization, the graphite tube is heated firstly by the wall. Therefore, in a standard graphite tube such as the high-density or the pyrolytic-coated tube, the sample is heated and atomized as the wall is heated. In a platform tube, however, the sample is atomized after the temperature of the entire tube reaches the atomization temperature, so the sample is atomized under optimum temperature distribution. When measuring with platform tube, the atomization peak becomes broader although the platform material is pyrolytic graphite (Figure 6).
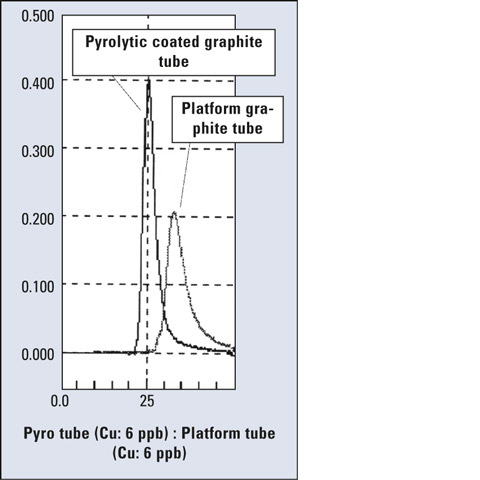 Figure 6: Comparison of peak profiles of pyrolytic-coated (6 ppb) and platform graphite tube (60 ppb)
Figure 6: Comparison of peak profiles of pyrolytic-coated (6 ppb) and platform graphite tube (60 ppb)
Platform tube is the effective solution for complex matrix samples
The platform tube requires the temperature for ashing and atomization to be set at a value of 100 °C to 200 °C higher than normal pyrolytic-coated tubes. The difference in the heating characteristics of the platform tube in comparison to a standard tube minimizes the influence of matrix effects of complex samples, so the background signal is separated clearly from the element signal, especially in combination with matrix modifiers such as palladium, iridium, rhodium and others. Use of the platform tube and matrix modification is therefore the most effective solution for the determination of elements in samples with complex matrix, such as biological sample, waste water and seawater.