PAHs: simple analysis with high detection limits
Determination of PAHs or mineral oils in water using fluorescence spectroscopy according to ASTM D 5412

What do drinking water and wastewater have in common? Contamination. With wastewater, it’s likely to think about the total sum of contamination, whereas with drinking water more detailed information on contaminations at trace level may be obtained. Contaminations in drinking water are considered to occur in the ppm concentration range or lower.
Various analytical techniques are available to address a wide range of analytical problems:
- Hydrocarbons (C5 – C44, and up to a chain length of C60) are chromatographically separated and determined quantitatively using GC analysis in accordance with DIN DEV 38409-H53 and ISO 9377-2.
- FTIR spectroscopy is still used, in part, to determine hydrocarbon mineral oils and aromatic hydrocarbons in drinking water in the ppm down to the ppb range (DIN 38409-H18, although this standard expires due to a ban on the solvents used)
- The total organic carbon content in drinking water and wastewater is determined using TOC analysis. The relevant TOC standard is EN 1484 ‘Water analysis – Guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC). This standard applies to a measurement range of 0.3 – 1,000 mg/L.
The techniques mentioned above are ideally suited for detection of impurities or contaminations in the lower ppb range. These techniques are subject to corresponding national and EU regulations.
Fluorescence spectroscopy: little sample preparation, fast results
An alternative analytical technique leading to fast results with little sample preparation is fluorescence spectroscopy. Because of its sensitive measurement principle, detection limits for contaminations in the lower ppb range are easily achieved. The regulation or the standard for this analysis has been established by the American Society for Testing and Materials (ASTM). The D 5412 standard testing method includes determination of complex polycyclic aromatic hydrocarbons (PAHs) or mineral oils in water [1].
The PAHs or mineral oils are quantified in aqueous samples using fluorescence spectroscopy. Appropriate calibration standards exhibiting similar emission and synchronous fluorescence spectra are used for calibration. According to the ASTM, this test method is suitable for contaminations from mineral oils, fuel oils and tar oils. Industrial organic contaminants cannot be excluded.
In accordance with the ASTM standard method, the PAHs or mineral oils are dissolved in cyclohexane. The initial concentration of an unknown sample should be approximately 100 µg/L for a fluorescence measurement. It may be necessary to dilute this solution in order to bring the fluorescence signal into the linear range and to suppress self-absorption effects of the matrix. Cyclohexane has been recommended as a suitable solvent for this analytical method. Alternative solvents can be substituted but they should comply with the ASTM requirements. The 10 mm layer thickness cuvette must be made of non-fluorescent quartz.
Representation of selective fluorescence-active organic compounds
Fluorescence spectroscopy enables representation and quantification of selective fluorescence-active organic compounds in mixtures. Fluorescence spectrometers allow measurement of excitation and emission spectra, which can be used for substance identification. When a mixture contains several fluorescence-active compounds, excitation of one compound can trigger excitation of a second compound resulting in decreased fluorescence for that compound. Synchronous scanning is used to minimize these overlapping effects.
 RF-6000
RF-6000
In synchronous scanning, the two monochromators in Shimadzu’s RF-6000 spectrofluorophotometer are scanned simultaneously. To obtain a standard fluorescence spectrum, the excitation wavelength is set to the desired analytical wavelength and the emission is measured over the UV-VIS measuring range. Under synchronous scanning conditions, the monochromators are scanned synchronously at a fixed wavelength interval offset. For example, an excitation wavelength of 220 nm is selected and the resulting fluorescence is measured from a wavelength of 230 nm. Synchronous scanning is thus carried out at an offset of 10 nm. The desired effect is that the measurement acts as a background correction of the fluorescence arising from ‘neighboring’ compounds. It is known that fluorescence always occurs with a slight time delay with respect to excitation to higher wavelengths as a stray light phenomenon.
In the example shown here, a mixture of five different PAHs was prepared. The goal is to show that the synchronous scan can represent one of the substances.
Response of the organic compounds to UV light
Figure 1 shows the fluorescence spectrum of a mixture of five compounds. An analytical wavelength of 300 nm was used for excitation of the solution. Most of the organic compounds containing chemical ring systems respond to the UV light by emitting fluorescence. The 300 nm excitation wavelength is not selective for this compound mixture.
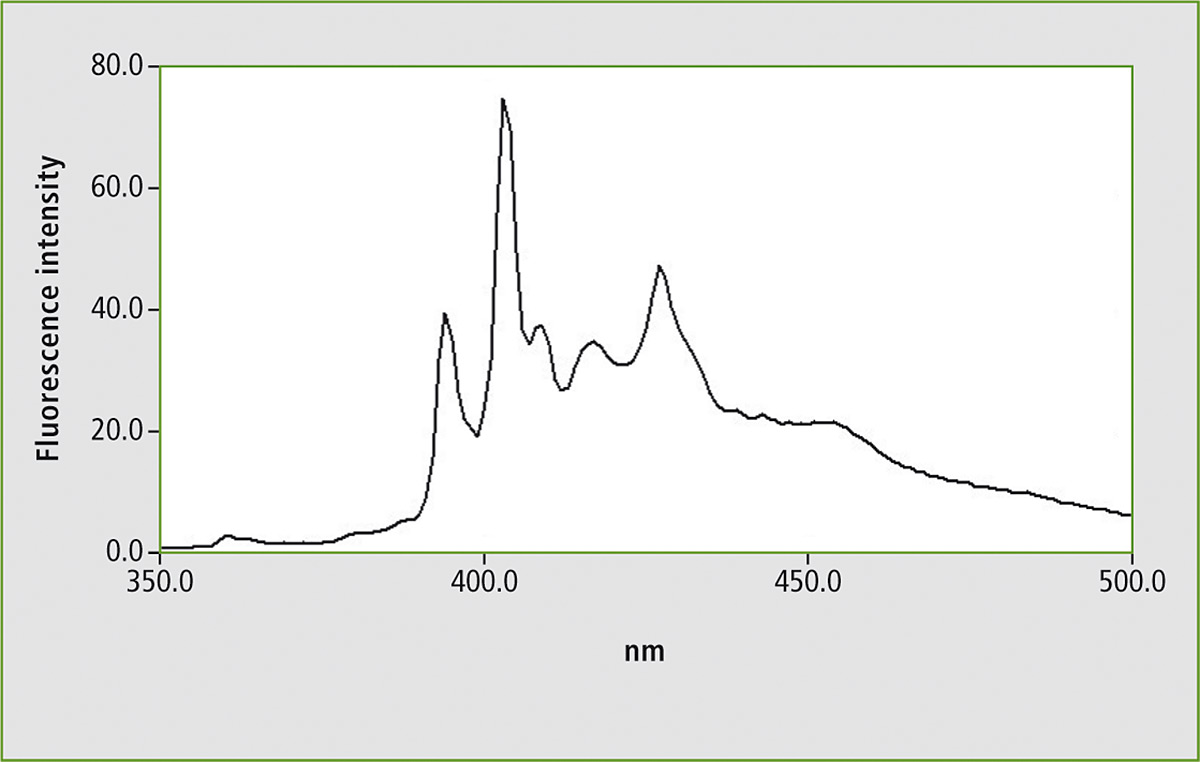 Figure 1: Fluorescence spectrum of a mixture consisting of five polycyclic aromatic hydrocarbons (PAHs) in a measuring range of 350 – 500 nm using an excitation wavelength of 300 nm
Figure 1: Fluorescence spectrum of a mixture consisting of five polycyclic aromatic hydrocarbons (PAHs) in a measuring range of 350 – 500 nm using an excitation wavelength of 300 nm
Some of the PAHs emit fluorescence following excitation of 300 nm. When the measurement is repeated using synchronous scanning, one signal group is isolated. Upon comparison of this result with a synchronous scan of benzo[a]pyrene, this PAH could be identified. Due to the distinct isolation of this PAH, it is also possible to quantify this substance.
Several examples of measurement of various mineral oils are described in the ASTM standard method. Figure 1 shows the fluorescence spectrum of the PAH mixture using a fixed excitation wavelength. Figure 2 shows the synchronous scan spectrum of benzo[a]pyrene at an offset of 6 nm. The isolation of benzo[a]pyrene from the mixture is shown in figure 3. This spectrum was also measured with an offset of 6 nm for comparison.
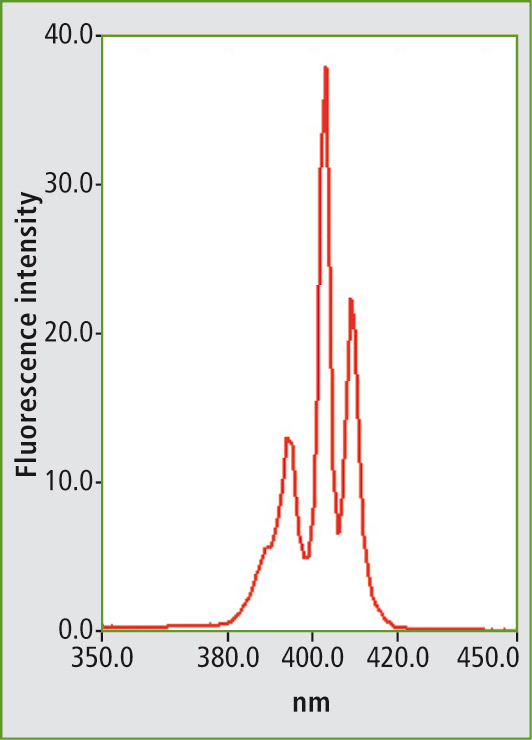 Figure 2: Synchronous scanning fluorescence spectrum of benzo[a]pyrene in a measuring range of 350 to 450 nm with an offset of 6 nm between excitation and emission wavelengths.
Figure 2: Synchronous scanning fluorescence spectrum of benzo[a]pyrene in a measuring range of 350 to 450 nm with an offset of 6 nm between excitation and emission wavelengths.
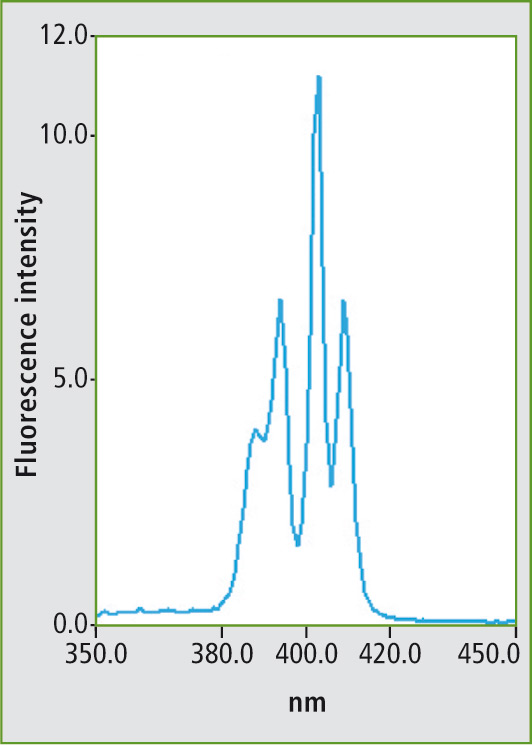 Figure 3: Synchronous scanning fluorescence spectrum of the mixture (fig. 1) in a measuring range of 350 to 450 nm with an offset of 6 nm between excitation and emission wavelengths
Figure 3: Synchronous scanning fluorescence spectrum of the mixture (fig. 1) in a measuring range of 350 to 450 nm with an offset of 6 nm between excitation and emission wavelengths
Conclusion
Fluorescence spectroscopy is highly suitable for the analysis of PAHs or mineral oils in aqueous samples. In the above-mentioned case, the benzo[a]pyrene spectrum could be obtained using synchronous scanning. Due to its high quantum yield, benzo[a]pyrene is detectable in low concentrations. In accordance with the ASTM standard, this method can detect concentrations of 0.5 ppm of oils in water. Detection limits are dependent on the individual fluorescence activities of the PAHs and the mineral oils.
Literature
[1] ASTM D 5412 – 93 (Reapproved 2000), Standard Test Method for Quantification of Complex Polycyclic Aromatic Hydrocarbon Mixtures or Petroleum Oils in Water