Complete solution for mycotoxin analysis
Mycotoxin Screening System analyzes ten different mycotoxins in only 14 minutes
It is hazardous to health, it is genotoxic and carcinogenic. It is one of the most dangerous substances in food: Aflatoxin B1. Of all types of aflatoxins, Aflatoxin B1 is the most common one present in food products [1]. It is part of mycotoxins, which are secondary metabolites produced by fungi [2]. Analysis of mycotoxins in food is therefore essential in order to assure healthy nutrition for humans and animals.
Aflatoxins are produced by fungal molds of the Aspergillus species, which prefer a warm and damp climate. They are often associated with commodities produced in the subtropics and tropics, like peanuts, cotton, spices or pistachios. Furthermore, they can be produced by fungal infestation during or after harvest of grain and can end up in processed products such as beer, brewed from malt.
Ochratoxin is produced by Penicillium and Aspergillus species. It is a contaminant in beverages such as beer and wine. Patulin, produced by P. expansum, Aspergillus, Penicillium and Paecilomyces fungal species, is often found in moldy fruits and vegetables. Zearalenone is produced by Fusarium species and may be present in crops; AFM1 is often found in milk products.
Mycotoxins are not destroyed by temperature treatment, and are barely influenced by cooking, freezing or digestion. These properties make the investigation in food crucially important as consumers need to be protected from these toxic substances. Sensitive methods for the analysis of mycotoxins are required.
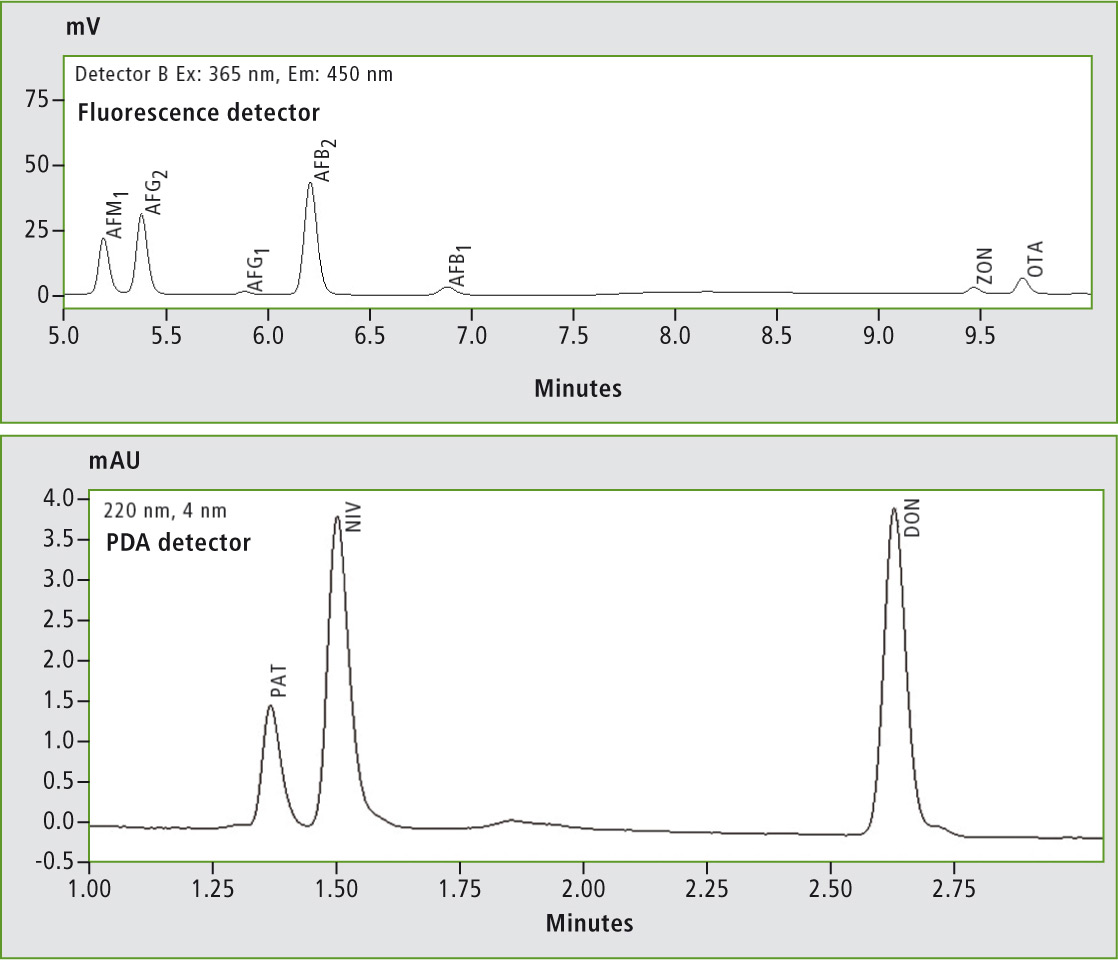 Figure 1: Mycotoxins detected by fluorescence and PDA detection
Figure 1: Mycotoxins detected by fluorescence and PDA detection
Complete solution
The Shimadzu “Mycotoxin Screening System i-Series Solution Package“ is a one package single solution. The system simultaneously investigates ten different mycotoxins in only 14 minutes. This i-series instrument specializes in identification of mycotoxins in grain, such as wheat flour and rice flour, as well as in apples and milk. Sample preparation, analysis and evaluation are easy comprehensible, explained progressively, and represent an easy-to-use solution for users.
Neither in-depth analytical nor chromatographic knowhow are necessary to operate the system. An additional benefit is the combination of a fluorescence detector with a PDA detector (Photo Diode Array). This prevents a complex derivatization, and the mycotoxins can be identified easily and efficiently.
AFB1, AFB2, AFG1, AFG2, AFM1, OTA, ZON, DON, NIV and PAT were investigated. For this approach, the five analytes most commonly tested in malt products were extracted and analyzed in spiked and non-spiked beer samples from different batches, as well as PAT in apple juice and grape juice. EU limit values are: Aflatoxins (B1, B2, G1 and G2: total: 4 to 15 µg/kg; AFB1,: 2 to 12 µg/kg); AFM1: 0.05 µg/kg; OTA: 2 to 10 µg/kg; PAT: 25 to 50 µg/kg; DON: 500 to 1,750 µg/ kg; NIV: not specified; ZON: 20 to 400 µg/kg. Detection of the mycotoxins was conducted using a combination of fluorescence and photodiode array (PDA) detection.
Investigation of mycotoxins in juice and beer
This method was applied to different non-spiked and spiked beverage samples. In all spiked beverage samples, the mycotoxins were successfully identified despite the complexity of the matrices. All ten mycotoxins were successfully separated from each other and detected by fluorescence detection and PDA
Analytical conditions
Instrument: LC-2040C 3D (Shimadzu)
Column: Shim-pack GIST C18 (3.0 mm x 75 mm I.D., 2 µm); Shimadzu
Mobile phase
A: 20 mmol/L sodium phosphate buffer pH 2.5 (10 mmol NaH2PO4 and 10 mmol H3PO4)
B: Acetonitrile
C: Methanol
Flow rate: 1.0 mL/min
Injection vol.: 10 µL
Ofen temp.: 55 °C
Detection
Fluorescence: RF-20AXS:
Ex: 365 nm and 320 nm
Em: 450 nm and 465 nm
PDA: D2 at 190-500 nm, Reference at 350 nm
Sample preparation
Two batches of beer, apple juice and grape juice were the object of this investigation. The samples were prepared as non-spiked or spiked with mycotoxins. 4 g of the beverages were mixed with 21 mL acetonitrile. The mixtures were cleaned with a multifunctional column (MultiSep 228, cartridge type). 4 mL of the elution were collected and evaporated to dryness with nitrogen gas. The samples were then re-dissolved in a 400 µL water/acetonitrile (95/5, v/v) solution and used directly for analysis. A standard mixture containing ten different mycotoxins (AFB1, AFB2, AFG1, AFG2, AFM1, OTA, ZON, DON, NIV and PAT) was prepared.
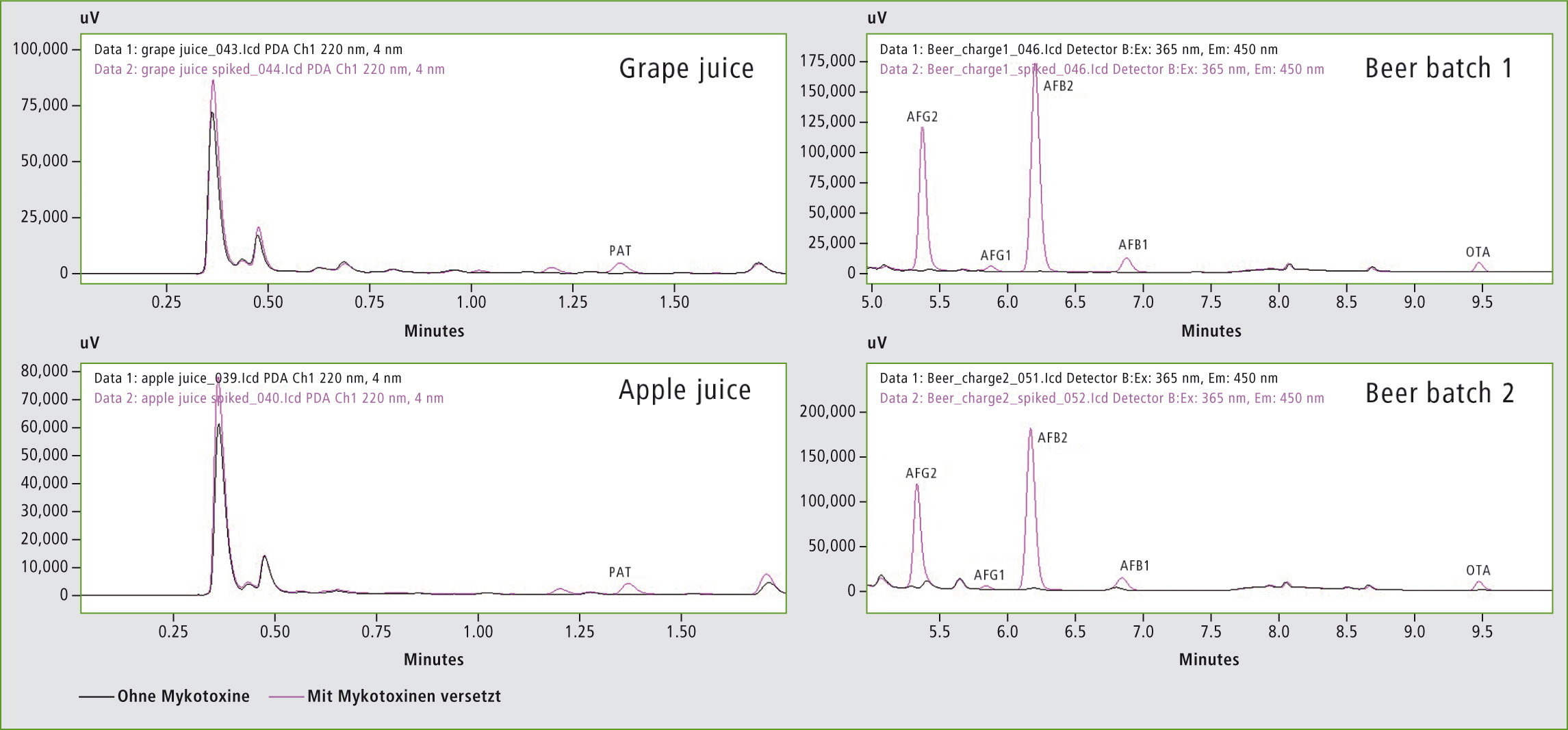 Figure 2: Comparison of pure and spiked beverage samples: apple juice, grape juice and two different batches of beer
Figure 2: Comparison of pure and spiked beverage samples: apple juice, grape juice and two different batches of beer
EU maximum residue limits, LODs and LOQs
The EU maximum residue limits for mycotoxins as specified by EU standards are the strictest in the world [3-5]. To check whether the food samples investigated contain mycotoxins within the EU maximum residue limits, standards with the mycotoxins were prepared using the same concentration as that of the EU control criteria. A simple and fast one point calibration was enough to assess compliance with the criteria.
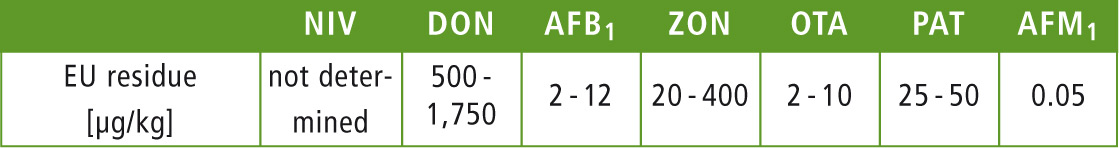 Table 1: EU residue limits for mycotoxins
Table 1: EU residue limits for mycotoxins
A batch was created with the calibration standard and the food samples, and the results show if the peaks of the mycotoxins in the food samples were below or above the criteria.
In this study, all measured samples (apple juice, grape juice and two batches of beer) either contained no mycotoxins or were in a range far below the EU criteria. The same, spiked samples showed mycotoxin concentrations above the EU criteria.
For all mycotoxin standards, LODs and LOQs were determined as shown in table 2. Limit of Detection (LOD) and Limit of Quantitation (LOQ) were low and at least half of the EU maximum residue limits.
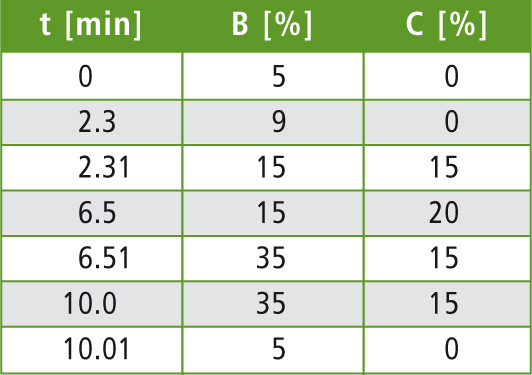 Table 2: LC gradient program
Table 2: LC gradient program
Figure 3 compares the different beer batches. The beer sample of batch 2 showed small peaks for AFG2, AFB2, AFB1, and OTA. Even though the concentration of the mycotoxins is far below the EU maximum residue limits, this is a clear indication of the presence of mycotoxins in the beer of batch 2.
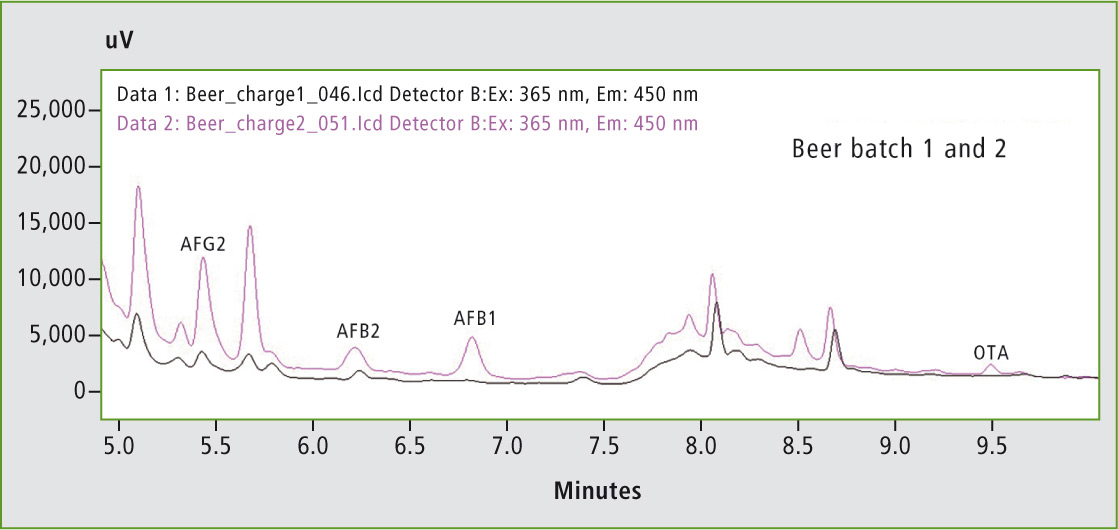 Figure 3: Comparison of non-spiked beer batch 1 and non-spiked beer batch 2
Figure 3: Comparison of non-spiked beer batch 1 and non-spiked beer batch 2
The limits of detection (LOD) as well as the limits of quantification of each mycotoxin have been defined and are listed in table 3. According to the regulations of sample preparation, LOD and LOQ are below the EU maximum residue limits.
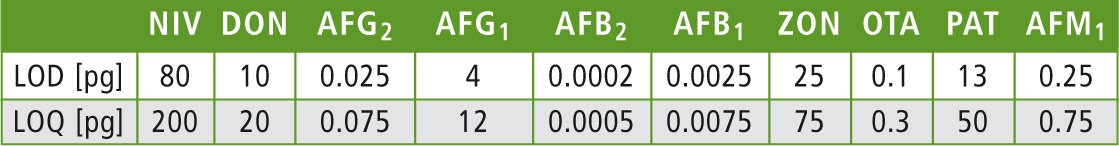 Table 3: Absolute LODs and LOQs of the mycotoxins, determined using fluorescence and PDA detection. Error for the LOQs was below 10 % for all analytes.
Table 3: Absolute LODs and LOQs of the mycotoxins, determined using fluorescence and PDA detection. Error for the LOQs was below 10 % for all analytes.
Conclusion
The level of these mycotoxins was below the European regulation. The results show that different batches of the same beer show differences in mycotoxin content. Within this study, a fast, safe and easy method was shown for the analysis of mycotoxins in beverages. A further advantage, especially for food quality control analysis, is the simultaneous separation of all ten mycotoxins in just 14 minutes.
Literature
[1] European Food Safety Authority
[2] Richard JL, J. Food Microbiol. 119 (1-2): 3-10 (2007).
[3] EU: Commission Regulation (EC) No 1881/2006 of 19 December 2006 (consolidated version 2010-07-01). Setting maximum levels for certain contaminants in foodstuffs.
[4] EU: Commission Regulation (EC) No 165/2010 of 26 December 2010 amending Regulation (EC) No 1881/2006. Setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins.
[5] EU: Commission Regulation (EC) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006. Setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A.