Acidic? Basic? Chelating? No problem for inert columns!
For reactive substances, inert LC columns are essential for a symmetrical peak shape
In any chromatographic separation, symmetrical peaks with an ideal Gaussian shape are preferable. Under standard conditions, this can usually be accomplished in case of neutral substances. However, the examination of strongly acidic, basic or chelating compounds often leads to so-called “peak tailing“ and thus to a clearly asymmetric peak shape. In these cases, the right choice of column is crucial: it should be as inert as possible for ionizable analytes in order to avoid undesired secondary interactions with the stationary phase.
The inertness of a column
What exactly is meant by “inertness“ of a column? Generally speaking, inertness means that as few undesirable interactions as possible take place between the column and the analyte.
When the three substance properties, acidic, basic and chelating have an asymmetric peak form (i.e. with undesirable interactions), it suggests that acidic substances show an insufficiently chemically bound phase or impurities that are contained in the phase and react with acidic substances. An insufficiently chemically bound phase contains free silanol groups. Unprotonated acids can compete with protons of protonated silanol groups. This generates asymmetrical peaks. Contaminants in the stationary phase that can trigger hydrogen bonds, or are basic, can interact with acidic analytes and lead to asymmetric peaks.
Positively charged basic analytes may interact with remaining slightly acidic silanol groups on the stationary phase if they have a negative charge. “Endcapping“, i.e. the blocking of accessible silanol groups by trimethylsilyl ligands, is carried out to reduce this interaction as much as possible.
Asymmetric peaks of chelating analytes indicate that metal is present on the phase which chelating groups can approach and potentially irreversibly bind. With the undesirable interactions described, strong tailing of the peak occurs which in the worst case can also lead to a decrease of the peak area.
Most importantly, a column with little inertness is not the same as a bad column! Whether or not inertness is desired is always specific to the application. For some analytes, particular polar interactions or metal interactions are advantageous to achieve stronger retention.
This article deals exclusively with analytes for which inert columns are important. Different column types were investigated with regard to their inertness and their interactions.
Measurement parameters and methods
Instrument:
LC-2040C 3D (Shimadzu)
Column:
Shim-pack GISS C18;
(100 mm x 2.1 mm I.D., 1.9 µm)
Shim-pack GIST C18;
(100 mm x 2.1 mm I.D., 2 µm)
C18 (Competitor column 1);
(100 mm x 2.1 mm I.D., 2,7 µm)
C18 (Competitor column 2);
(100 mm x 2.1 mm I.D., 2.6 µm)
Shim-pack GIS RP-Shield;
(150 mm x 4.6 mm I.D., 5 µm)
Shim-pack GIST C18-AQ;
(100 mm x 2.1 mm I.D., 1.9 µm)
Shim-pack GIST Phenyl;
(100 mm x 2.1 mm I.D., 3 µm)
Shim-pack GIST Phenyl-Hexyl;
(100 mm x 2.1 mm I.D., 3 µm)
Mobile Phase:
Acid Test:
75 % 0.1 % H3PO4; 25 % ACN
Chelation test:
60 % 0.1 % H3PO4, 40 % ACN
Basic test:
65 % 20 mmol KH2PO4, pH 6,9, 35 % ACN
Furnace temperature: 40 °C
Flow rate and injection volume were adapted to the column dimensions.
Results
Differences in the measurement results of the column tests with regard to inertness were observed. The highest inertness was shown by the Shim-pack GISS C18 and the Shim-pack GIST C18. Inertness is one of their greatest advantages. The chromatograms in figures 1 and 2 demonstrate that all peaks are symmetrical and have a high intensity.
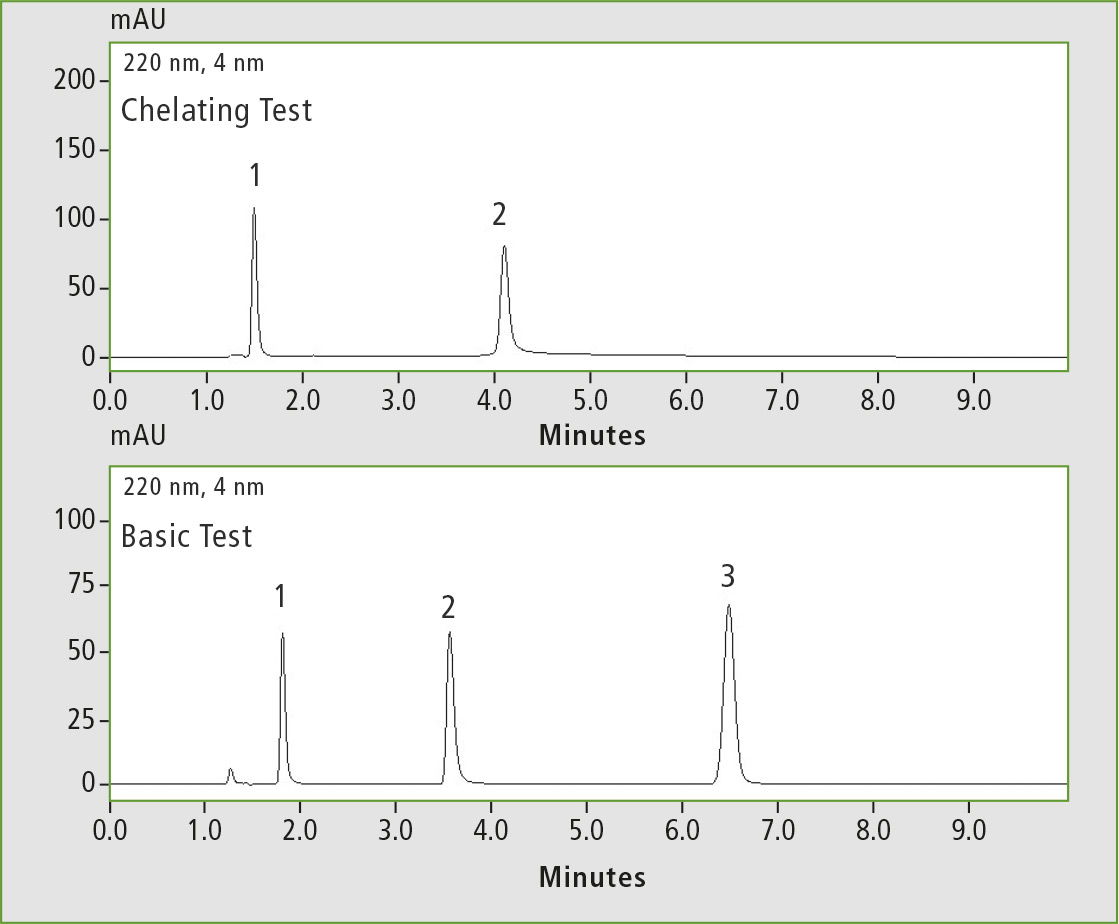 Figure 1: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the Shim-Pack GISS C18
Figure 1: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the Shim-Pack GISS C18
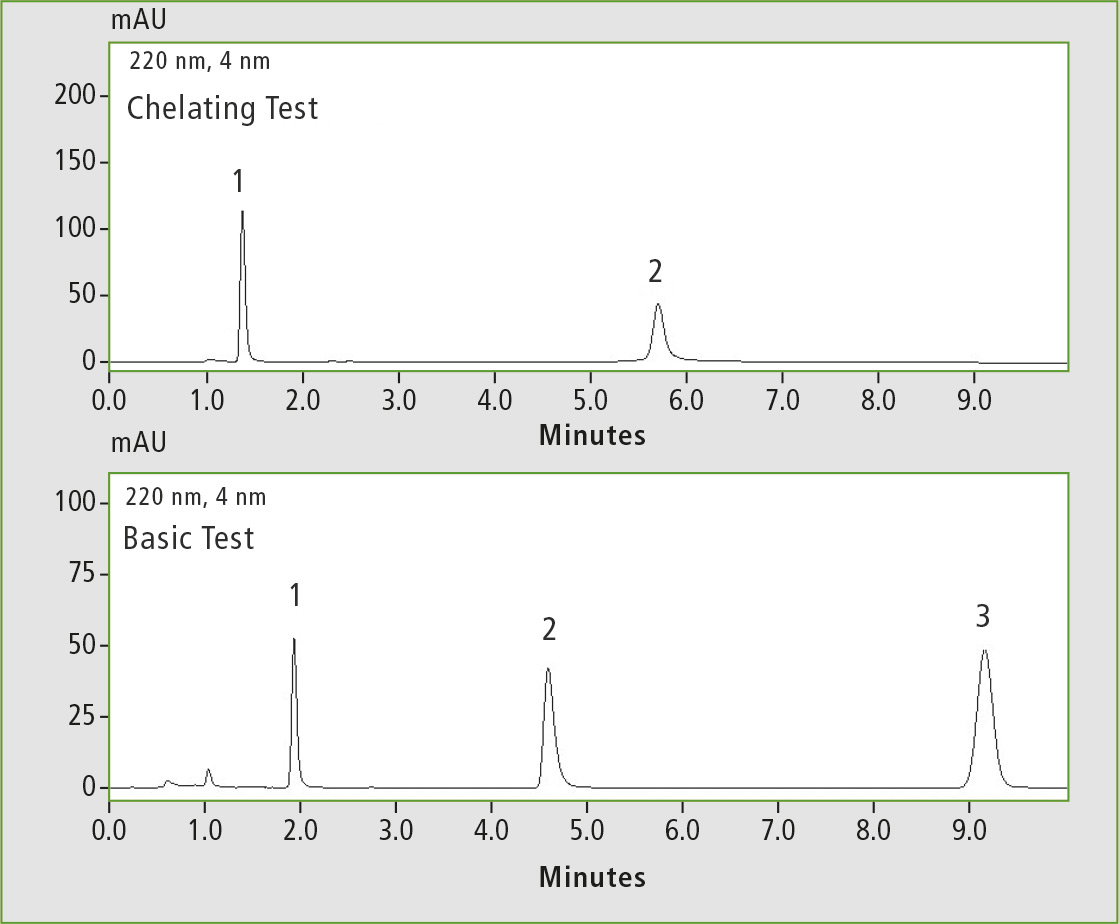 Figure 2: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the Shim-Pack GIST C18
Figure 2: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the Shim-Pack GIST C18
This illustrates an extraordinary inertness in the analysis of reactive substances, e.g. strongly acidic, basic or chelating analytes. Figures 3 and 4 illustrate comparative measurements on two different competitor C18 columns. Compared with figures 1 and 2, the chelating agent hinokitiol and the basic substance dextrometorphan show clear peak tailing and a lower intensity.
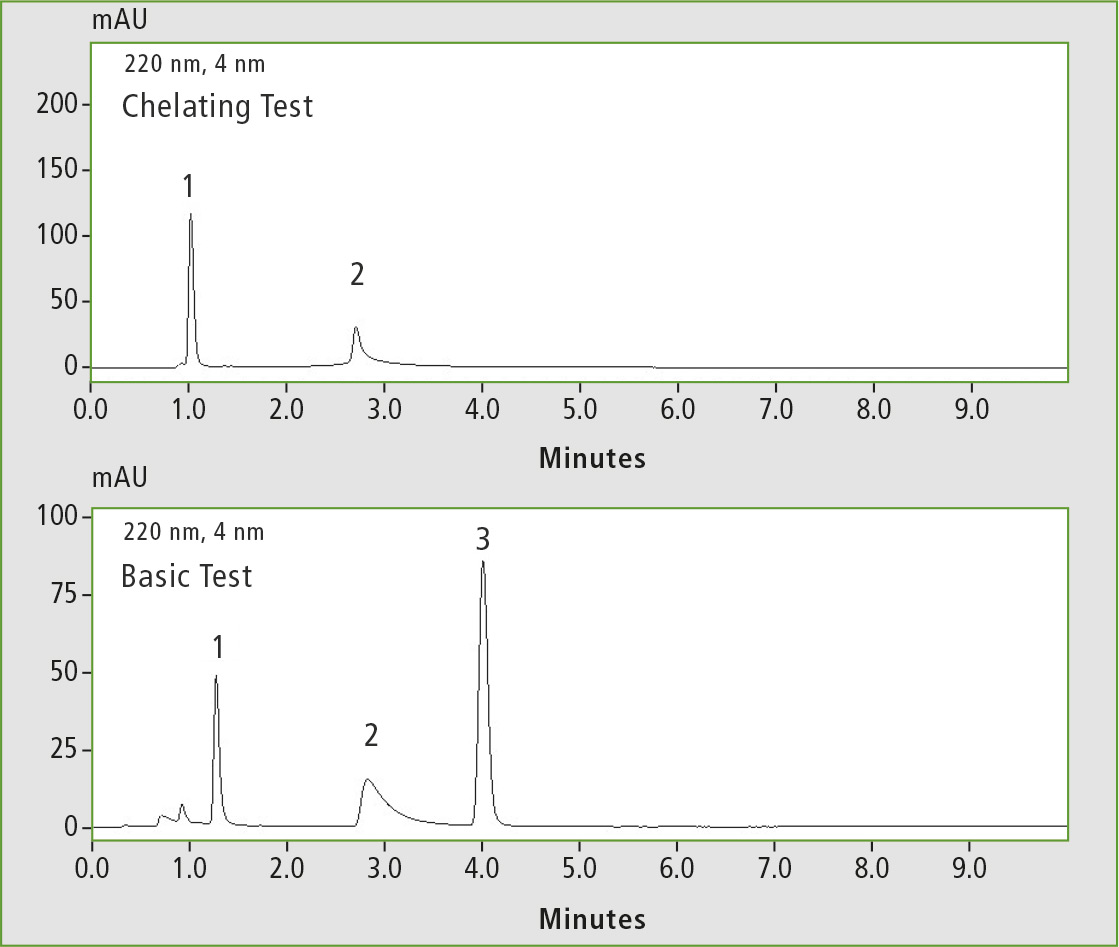 Figure 3: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the C18 competitor column 1
Figure 3: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the C18 competitor column 1
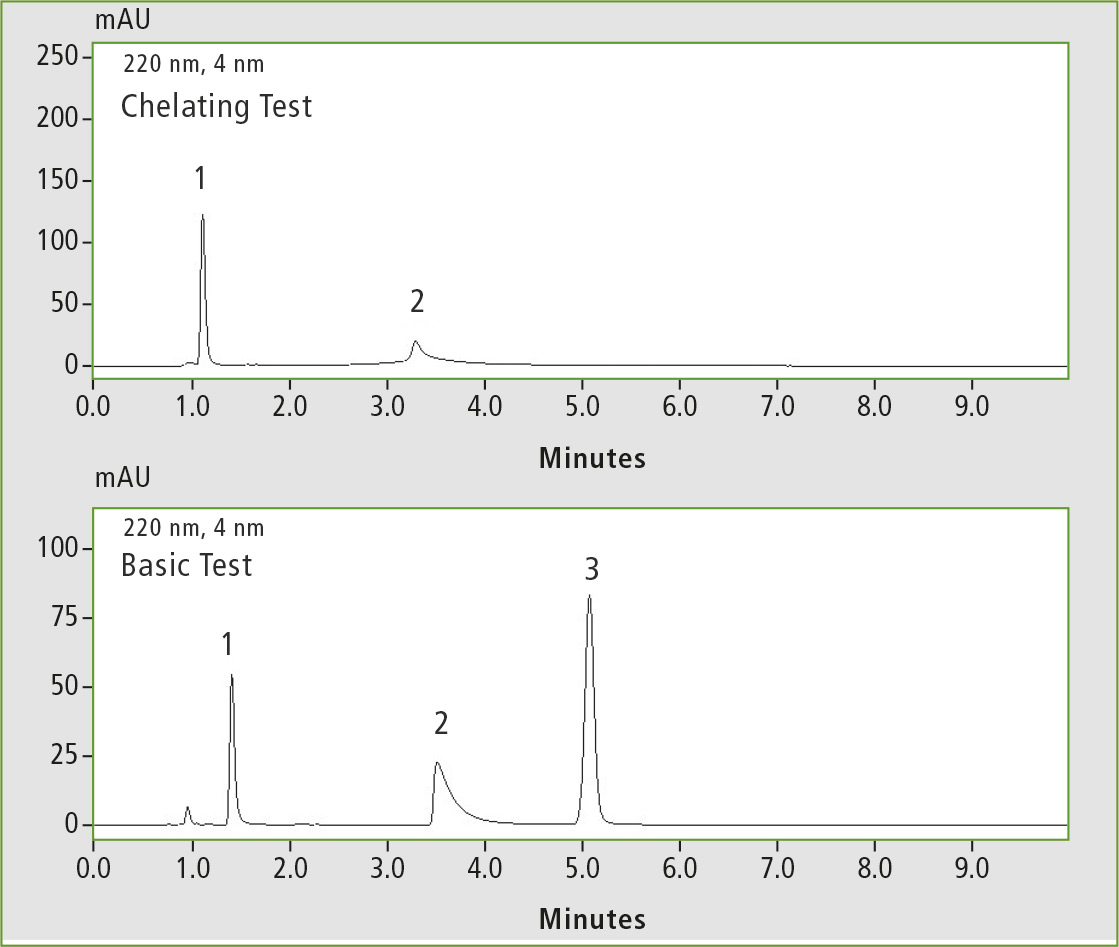 Figure 4: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the C18 competitor column 2.
Figure 4: Chelating test (1 caffeine, 2 hinokitiol) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the C18 competitor column 2.
The GIS RP-Shield column has a polar functional group embedded between the silica surface and the C18 groups. This allows stability even under 100 % aqueous conditions.
In the column test, this property is clearly visible in the chromatogram in the basic test, since the peaks, especially the dextrometorphan peak, are misshapen and asymmetrical (figure 5). On the other hand, for acidic analytes which can be measured ideally with this column, symmetrical peak forms are obtained.
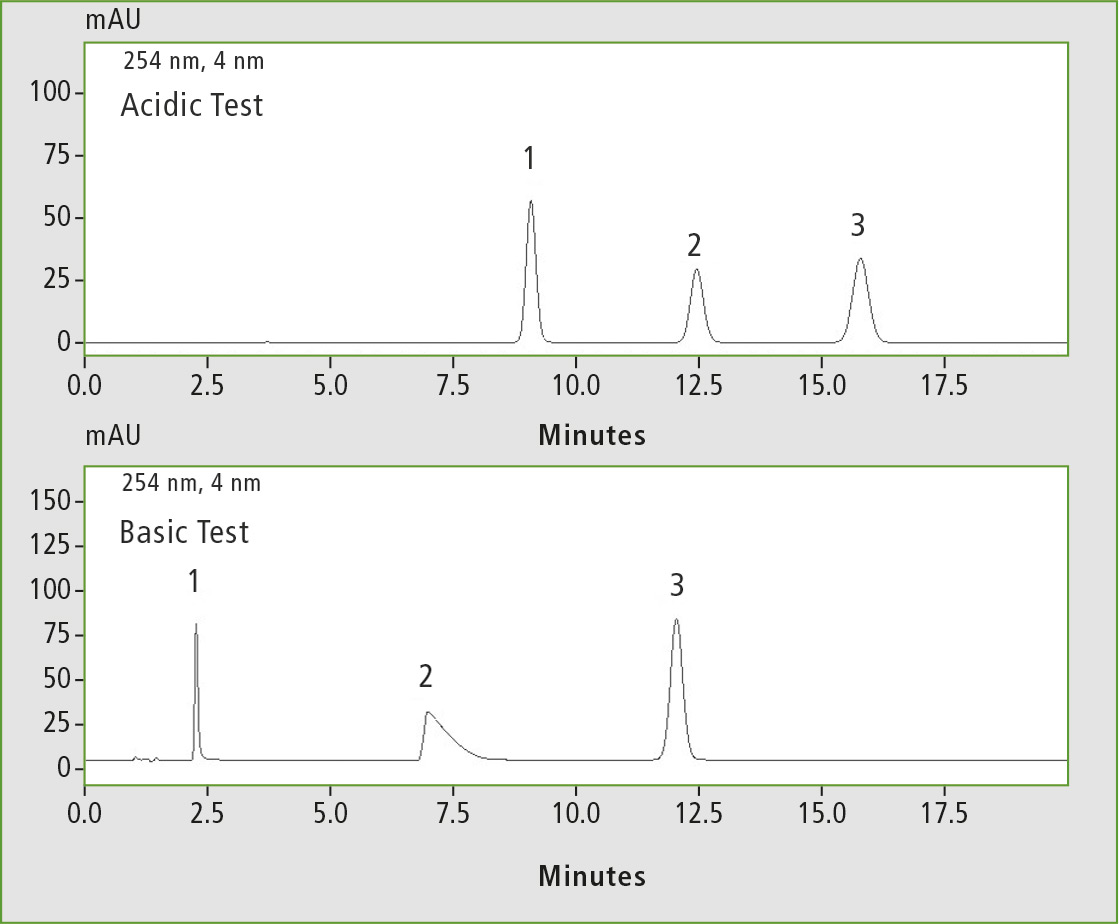 Figure 5: Acidic test (1 Salicylic acid, 2 Methyl parabene, 3 Cinnamic acid) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the Shim-Pack GIS RP-Shield
Figure 5: Acidic test (1 Salicylic acid, 2 Methyl parabene, 3 Cinnamic acid) and basic test (1 pyridine, 2 dextro-metorphan, 3 propyl paraben), measured on the Shim-Pack GIS RP-Shield
Compared with the phenyl-hexyl column, the phenyl column has no endcapping and therefore offers more polar interactions. This can be seen in the chromatograms in figures 6 and 7 when comparing the analyses of the basic substances on both columns.
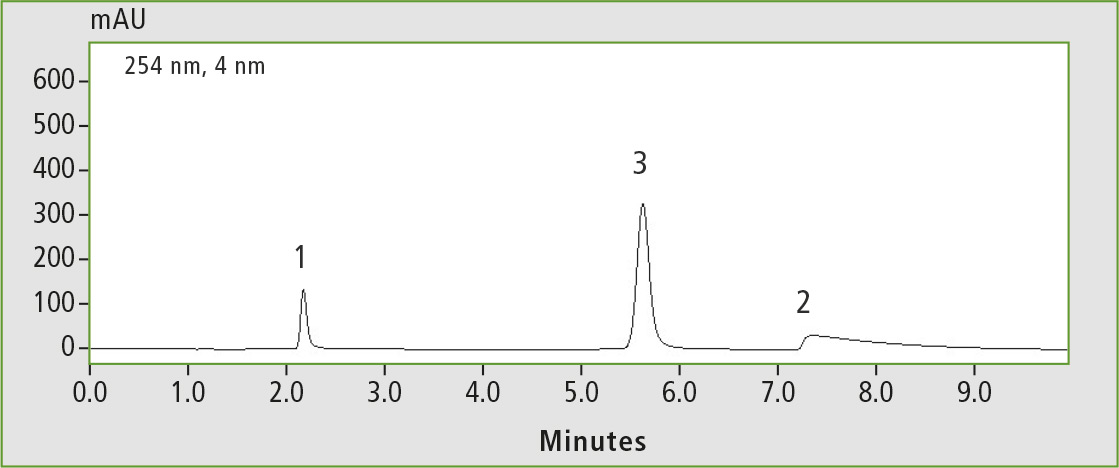 Figure 6: Basic test (1 pyridine, 2 dextrometorphan, 3 propylparaben), measured on the Shim-pack GIST Phenyl
Figure 6: Basic test (1 pyridine, 2 dextrometorphan, 3 propylparaben), measured on the Shim-pack GIST Phenyl
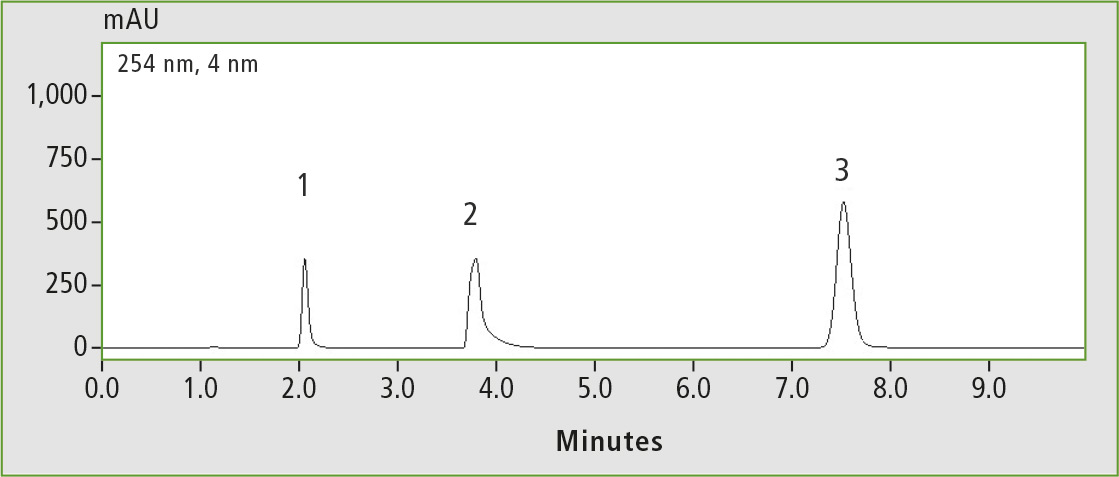 Figure 7: Basic test (1 pyridine, 2 dextrometorphan, 3 propyl paraben), measured on the Shim-pack GIST phenyl-hexyl
Figure 7: Basic test (1 pyridine, 2 dextrometorphan, 3 propyl paraben), measured on the Shim-pack GIST phenyl-hexyl
Dextrometorphan shows strong tailing on the phenyl column compared to the phenyl-hexyl column; the elution order is even reversed. This reversal is caused partly by the different functional groups of the phenyl and phenyl-hexyl columns.
Compared to conventional C18 columns, the GIST C18 AQ column shows very good retention to hydrophilic polar analytes. It can also be used with a 100 % aqueous mobile phase without loss of retention. The C18 AQ column is very inert. It shows tailing of basic substances due to the free silanol group present on more polar phases such as C18 AQ (figure 8).
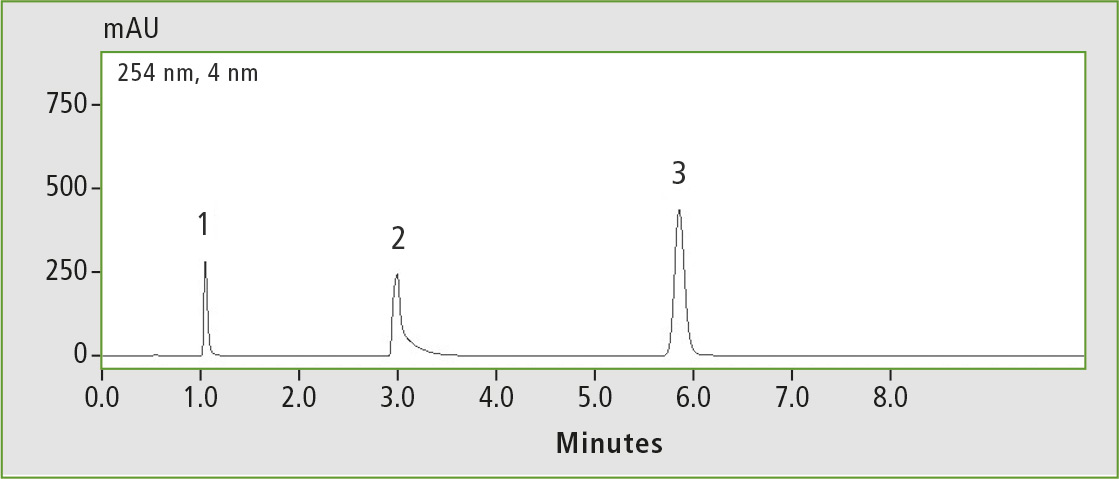 Figure 8: Basic test (1 pyridine, 2 dextrometorphan, 3 propyl paraben), measured on the Shim-pack GIST C18-AQ
Figure 8: Basic test (1 pyridine, 2 dextrometorphan, 3 propyl paraben), measured on the Shim-pack GIST C18-AQ
Conclusion
For the analysis of strongly acidic, chelating or basic substances, an inert column must be used. In the Shimadzu range, GIST C18 and GISS C18 show particularly inert properties. Substances can be measured accurately with these two inert columns and show symmetrical peaks with a high intensity.
If polar interactions are desired in order to obtain an alternative selectivity for analytes, the choice should fall on one of the different columns with polar interactions in the Shim-pack range.