Short on helium? Consider the carrier-gas alternatives
Short on helium? Consider the carrier-gas alternatives
The advantages of using hydrogen and nitrogen in gas chromatographic measurement
Helium is a preferred carrier gas in gas chromatography because of its high inertness and abilities supporting chromatographic separation. Demand for helium has been increasing, but limited availability and current events have exacerbated a pre-existing helium shortage. Prices therefore continue to rise, forcing users to look for alternatives. Nitrogen and hydrogen offer great potential to GC users. Both have drawbacks, but together with flexible gas-type selection and reduction of consumption they offer the key to success.
The role of helium in gas chromatography
In gas chromatography (GC), the carrier gas transports the sample from the injection system via the separation column to the detector. Helium is a preferred carrier gas due to its high inertness and good separation abilities. However, it is a limited natural resource, and demand has been rapidly increasing for use in ever more applications in the medical, scientific, and industrial fields. Current circumstances have restricted supplies and lengthened delivery times, resulting in a helium shortage. This has led to rising prices, and some labs have been having a hard time getting any helium at all.
Given these conditions, what are gas chromatography users to do?
What are the choices?
Historically, inert gases were commonly used for gas chromatographic systems as they are easy and safe to handle. Helium and nitrogen were the most popular choices available among the inert gases.
Nitrogen has a sufficient inertness for most gas chromatographic techniques, but it has a lower diffusivity compared with helium or hydrogen. This restricts the use of nitrogen as a carrier gas in applications with challenging separation.
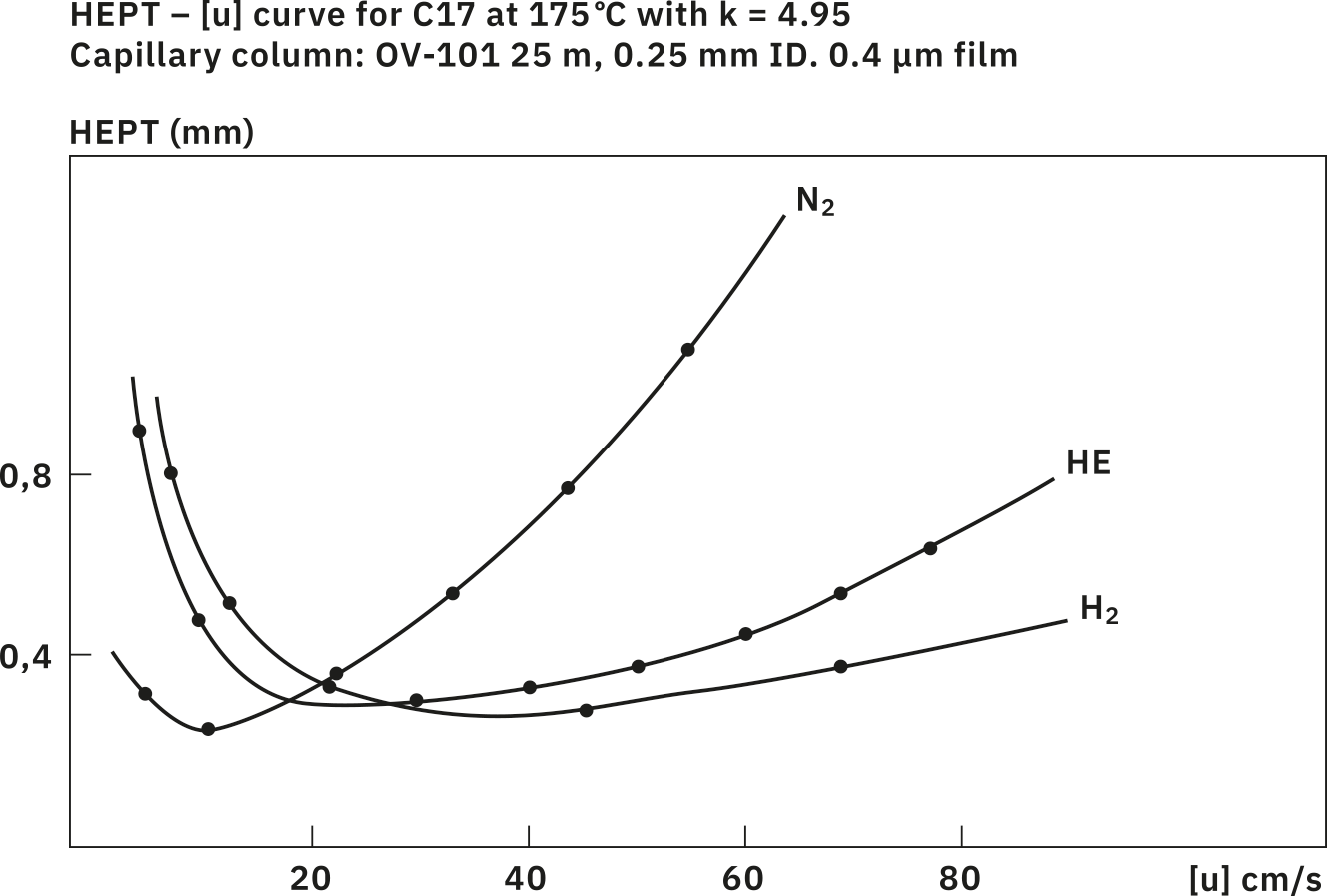
Gas chromatographic separation depends much on the carrier gas choice. The Van Deemter curves show the height equivalent to the theoretical plate (HETP) in relation to the linear velocity of the gas (Fig. 1). The lower the HETP value, the better the chromatographic separation is. The use of nitrogen as a carrier gas is limited, as the optimum linear velocity value is quite low, leading to long analysis times. With higher velocities, the nitrogen curve rises rapidly, which means that the peak resolution in a chromatogram gets worse.
In contrast, helium allows higher linear velocities, thus offering the possibility to decrease chromatographic runtimes significantly when compared with nitrogen. This and its inertness have made it the most popular carrier gas for GC analysis to date.
Hydrogen is the best alternative to helium with respect to gas chromatographic separation. It is a highly efficient carrier gas that maintains its separation efficiency across a wide linear velocity range. It is also a good choice when speeding up analysis times by using ‟fast GC” techniques. But the main advantage of hydrogen over helium is its low viscosity. For same-flow or linear velocity rates, helium requires almost a double-pressure gradient over a capillary column. In addition, hydrogen has no supply limitations, as it can be mass-produced by gas generators.
That said, hydrogen is reactive and bears an explosion risk if the concentration rises to 4% in the air. So strict precautions for safe operations with the gas must be taken. In addition, hydrogen is not always an appropriate carrier gas, in particular in combination with some detection systems or advanced techniques used in gas chromatography.
Using nitrogen without compromising performance
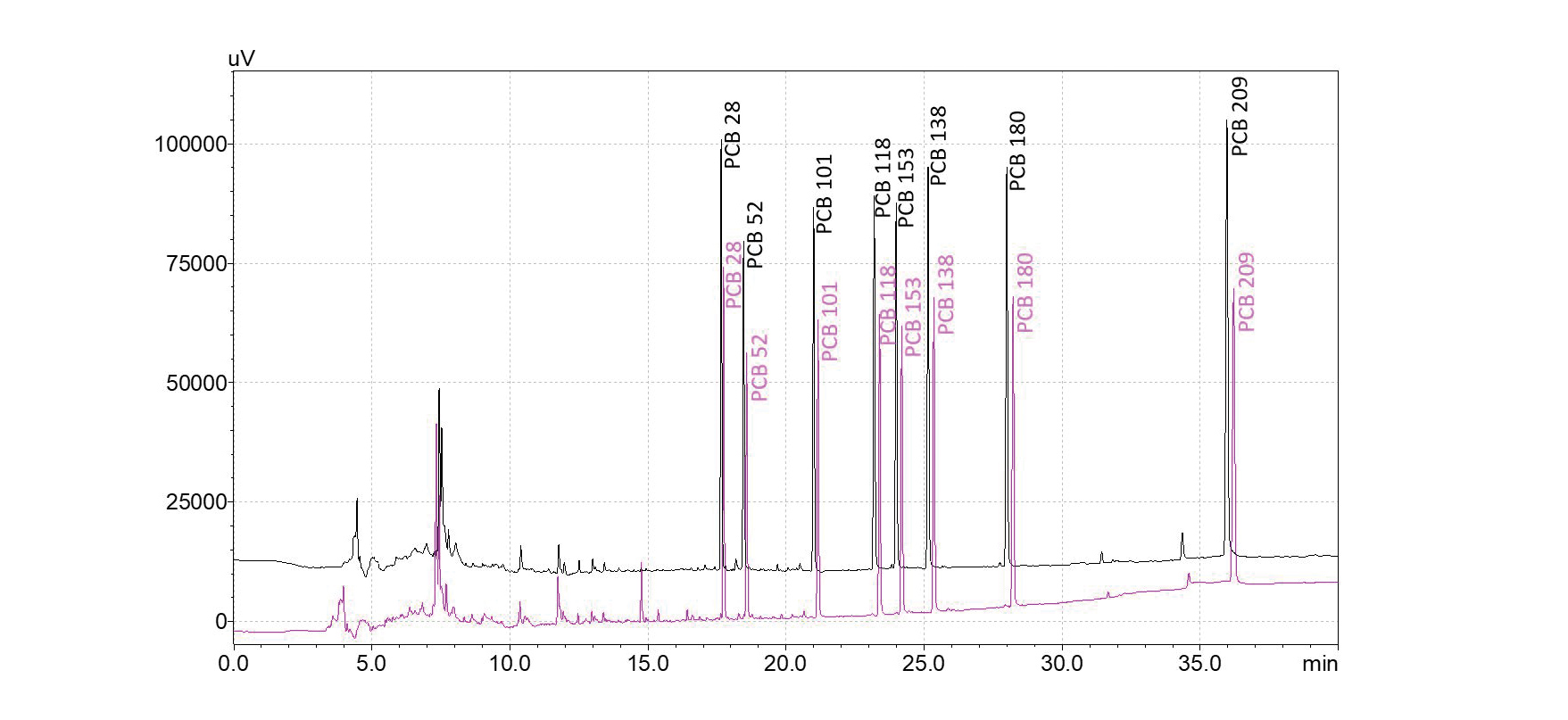
Typical, conventional GC analysis times using helium as a carrier gas range from 15 to 40 min, depending on the number of compounds, the separation power needed, and the matrix bakeout to prevent carryover. An application example at the higher end of this time range is the quantification of polychlorinated biphenyls (PCBs) in oil according to DIN EN 12766-1 and DIN EN 12766-2. A method using helium carrier gas and an SH-I-5 MS column (60 m, 0.25 mm ID, 0.25 µm df) that is sufficient for compound identification and separation has a runtime of 40 min at a linear velocity of 23 cm/s. Direct method translation to nitrogen carrier gas can be achieved, keeping compound separation and chromatographic runtime constant (Fig. 2).

Runtime benefits using hydrogen
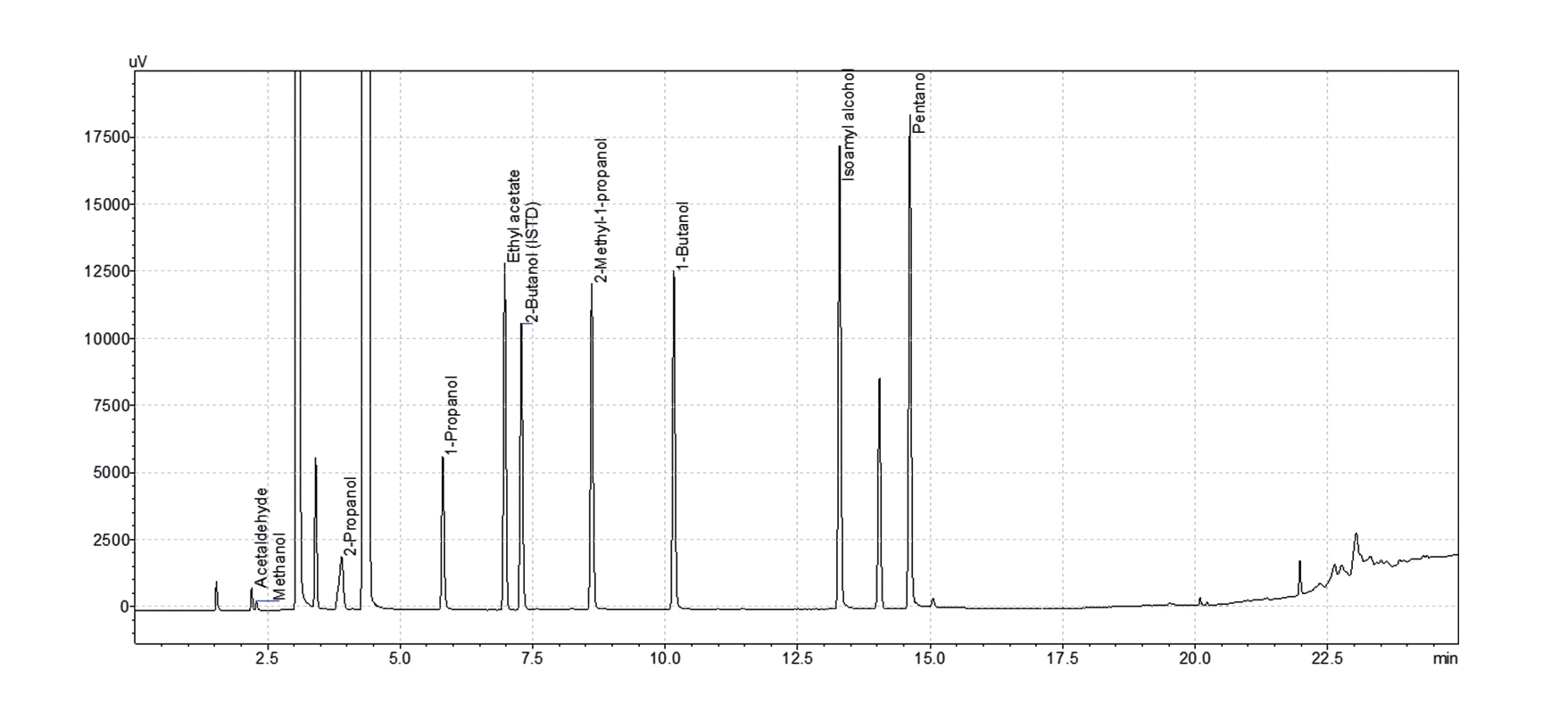
Another example with a runtime in the range of conventional gas chromatography using helium is the analysis of higher alcohols and ethyl acetate in alcoholic beverages. The alcoholic beverages are extracted using dichloromethane and the extracts then injected into the gas chromatograph. The last eluting target compound is pentanol, showing a retention time of 14.6 min on an SH-I-624 Sil MS column (30 m, 0.25 mm ID, 1.4 µm df). Yet, despite extraction, the remaining matrix elongates the runtime to around 24 min, limiting sample-throughput to two per hour (Fig. 3).
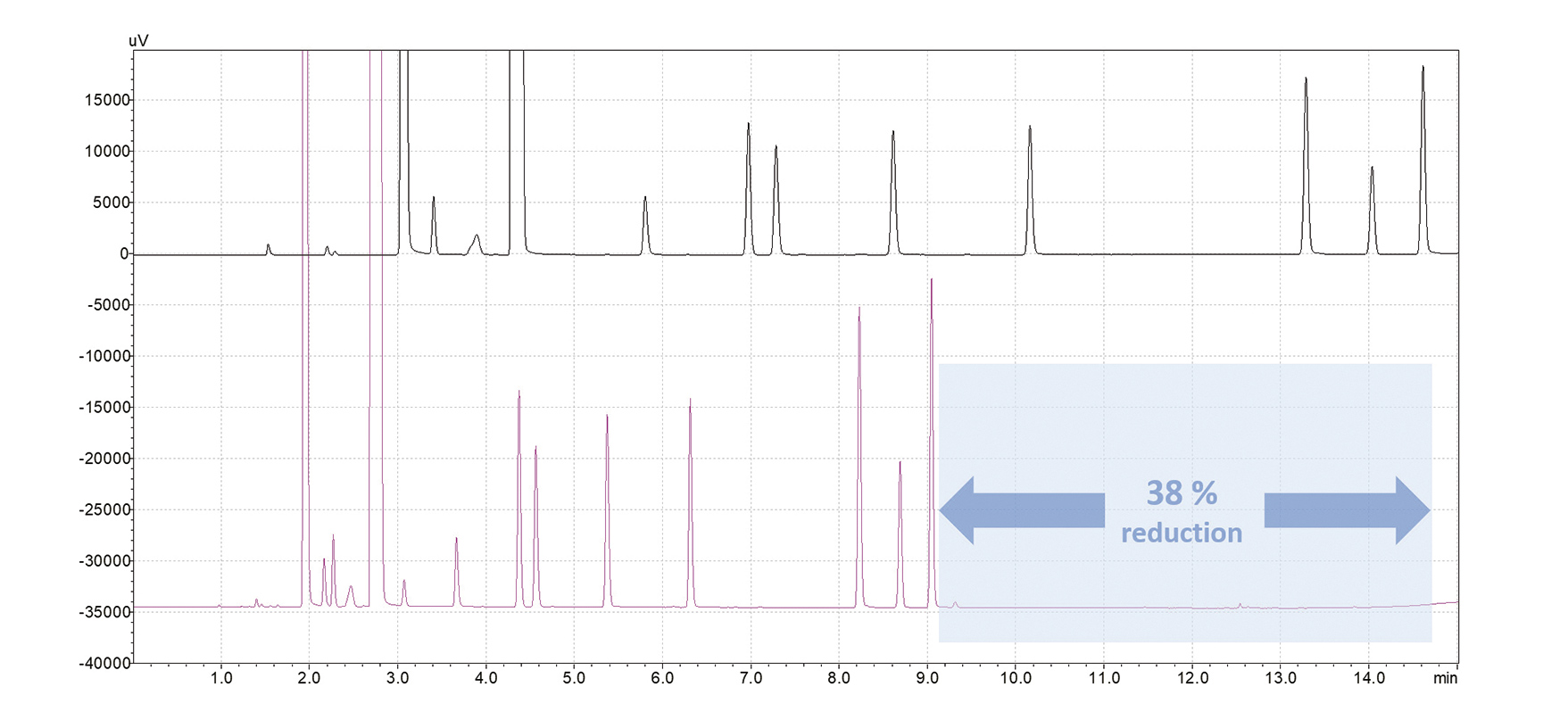
When using hydrogen as the carrier gas however, analysis runtime can be drastically reduced due to a higher linear velocity and an adjusted oven temperature program. Pentanol now shows a retention time of only 9.1 min, giving a time saving of 38%, which allows three samples per hour to be processed (Fig. 4). Separation efficiency is not compromised by this shorter chromatographic runtime, as demonstrated by the resolution between acetaldehyde and methanol: With helium carrier gas it is calculated to be 1.4, whereas hydrogen shows a value of 1.2, still ensuring reliable results (Fig. 5). In summary, switching from helium to hydrogen as carrier gas can provide significant time benefits, even for already short analysis times.
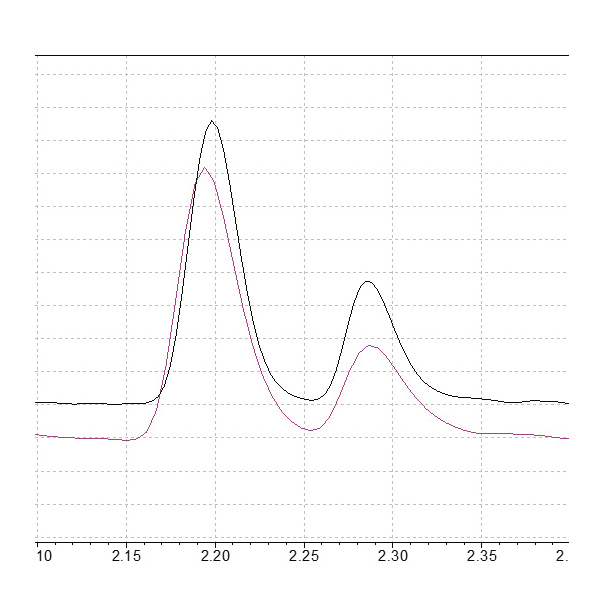
The analysis of mineral oil content in water according to EN ISO 9377-2 involves the monitoring of all compounds in the boiling point range of 175 °C to 525 °C. This describes all peaks found between the retention time markers n-decane and n-tetracontane. As it is not required to assign individual substances due to the complexity of the hydrocarbon mixtures, the analysis can be performed with a high linear velocity (60 cm/s). Using an SH-MetalX-1 column (15 m, 0.25 mm ID, 0.1 µm df), runtimes around 9 min can be achieved with helium as the carrier gas. However, switching to hydrogen allows an even higher linear velocity (80 cm/s) which, in combination with an adjusted oven temperature program, leads to a chromatographic runtime below 6 min. This results in a decrease in retention time for n-tetracontane of 30%, from 7.8 min to just 5.4 min (Fig. 6), allowing injection cycle times of 10 min.
Using hydrogen safely in GC
Despite its efficiency as a carrier gas, hydrogen remained less popular than helium for decades due to its lack of inertness. Hydrogen is a flammable gas that can potentially explode in air in a concentration range from 4% to 75%. Even though such concentrations are unlikely to be reached on a whole-laboratory level, a leak inside the GC oven – possibly due to column breakage close to the inlet – has the potential to reach an explosive level inside the system.
To ensure that this does not occur, the Shimadzu Nexis GC-2030 offers a three-pillar safety concept that supports the safe use of hydrogen as a carrier gas. A preventative, automatic leak-check function helps the operator to check for possible leakages after any kind of column maintenance. In the case of severe carrier-gas leakage occurring during standby or runtimes, the fast-responding advanced flow controller (AFC) immediately shuts off the carrier-gas supply.
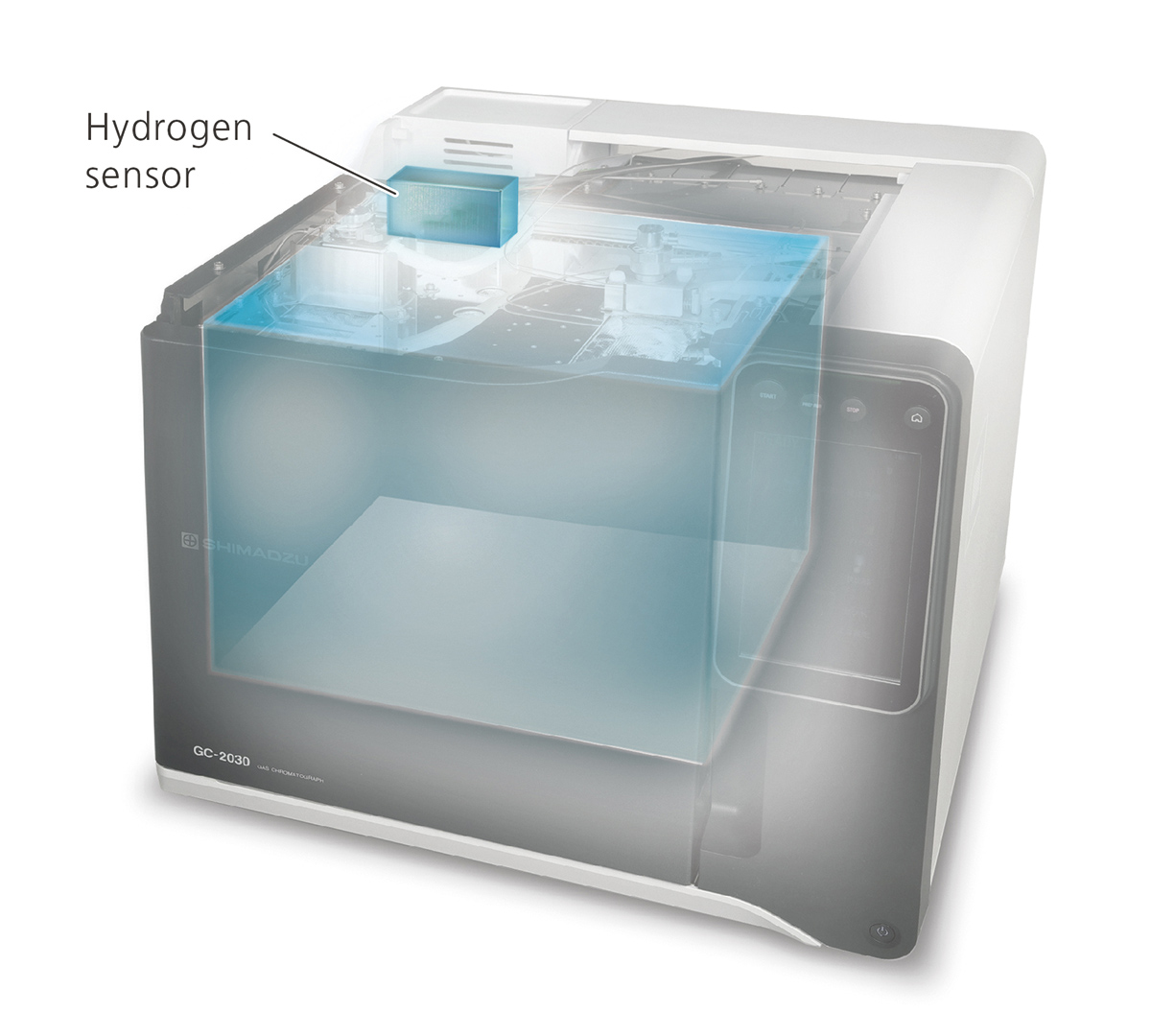
To cope with the risks resulting from less severe leakages or breakages of the capillary column during operation, independent hydrogen sensors for checking the air inside the column oven are available (Fig. 7). The basic sensor shuts off the carrier gas control once a level of 1% of hydrogen in air is reached. The advanced sensor model monitors the hydrogen level constantly and switches the gas control to an inert gas once the 1% concentration level limit is reached.
Cutting costs by reducing helium consumption
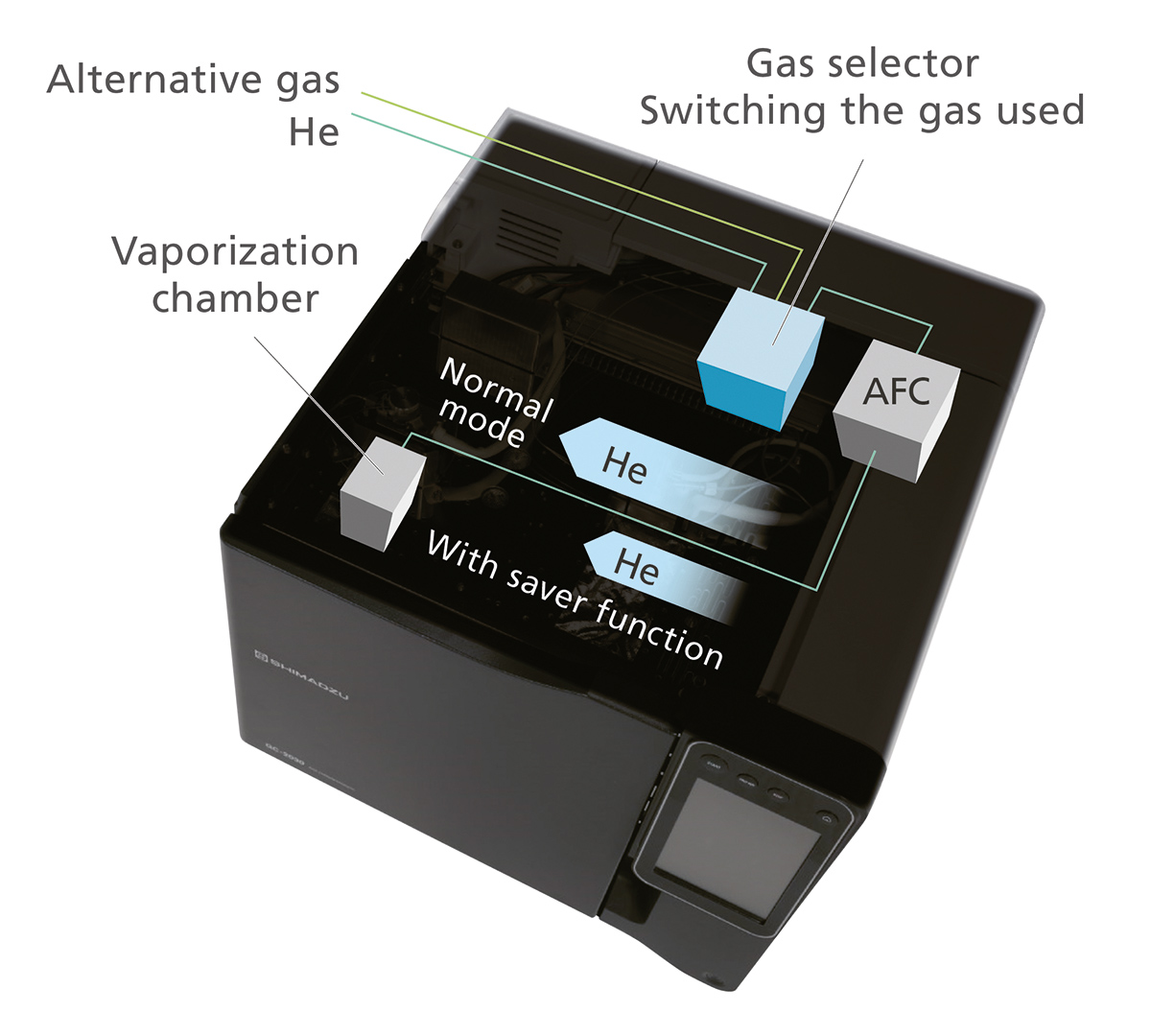
Helium is expensive, so users interested in efficient gas usage and instrument utilization need to consider both the active analysis times of a gas chromatograph and instrument standby times. To help reduce costs, the Nexis GC-2030 supports an automated gas selector for convenient gas switching between analyses and for standby times, facilitating the use of different gas types on the same instrument. This can be used for carrier and/or make-up gas selection and offers the choice between two gases that are simultaneously connected to the selector inlets (Fig. 8).
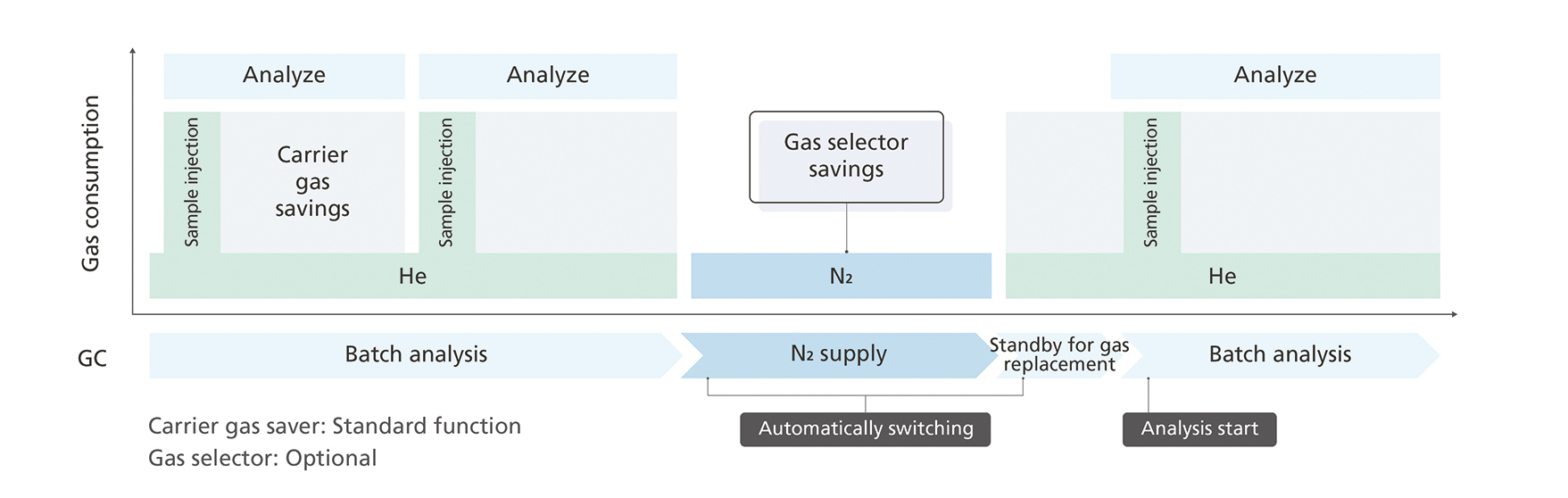
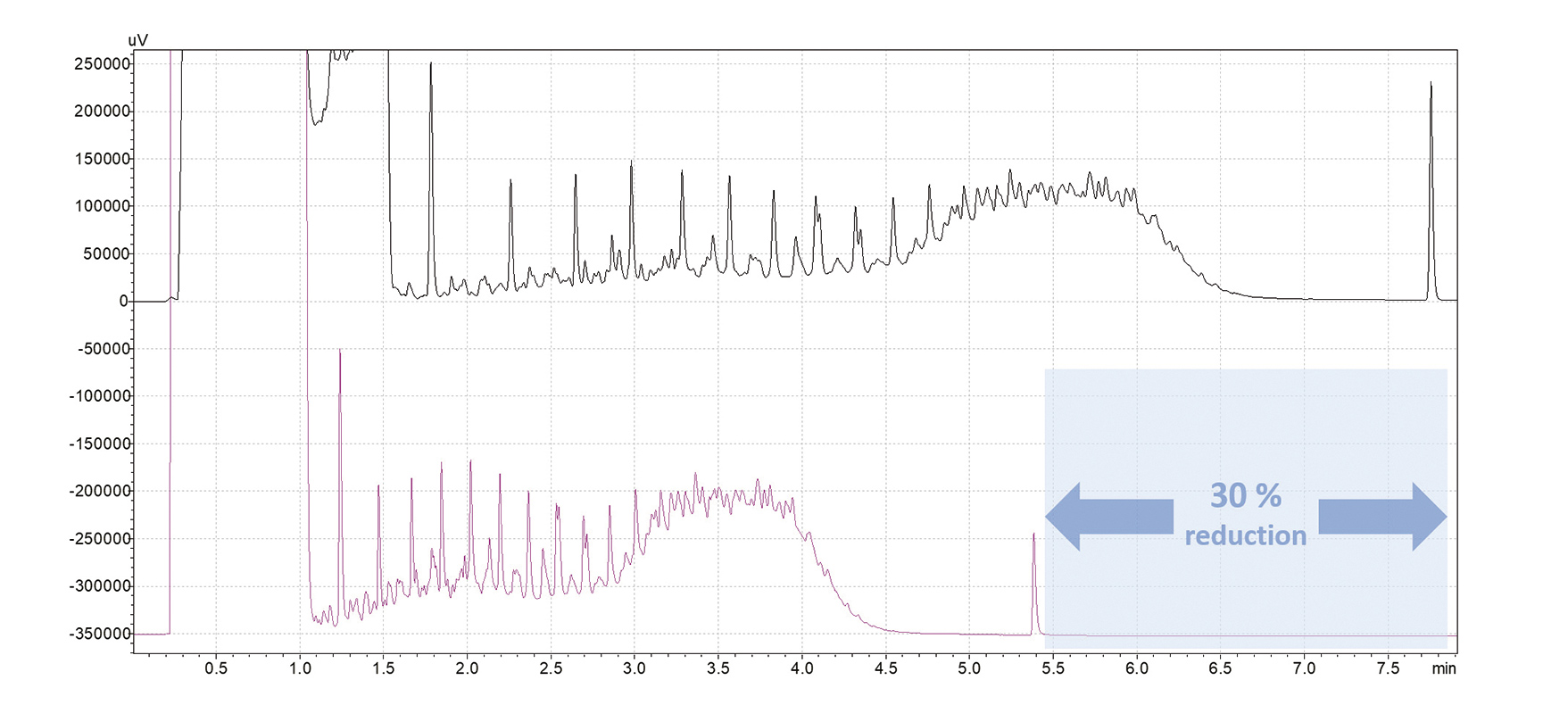
Automatic switching to nitrogen carrier gas for standby times after a sample sequence with helium significantly reduces helium consumption, even for applications where helium use is inevitable (Fig. 9). In combination with the carrier gas-saver functionality of the Nexis GC-2030 – which reduces the gas flow during analyses after injection and during brief standby periods – the overall gas savings can reach up to 90%, depending on standby times and analytical conditions.
The gas selector functionalities are fully software-controlled and gas choice for analysis is achieved simply by method choice. Supply gas settings are recorded in the acquired data, ensuring data integrity. An adjustable waiting time during the switching event guarantees full substitution of the previous gas to assure reproducibility of the results. And, if changing to another carrier gas type is not possible, helium consumption can still be significantly reduced using features offered by Shimadzu.
Optimising workflow with smart automatic features
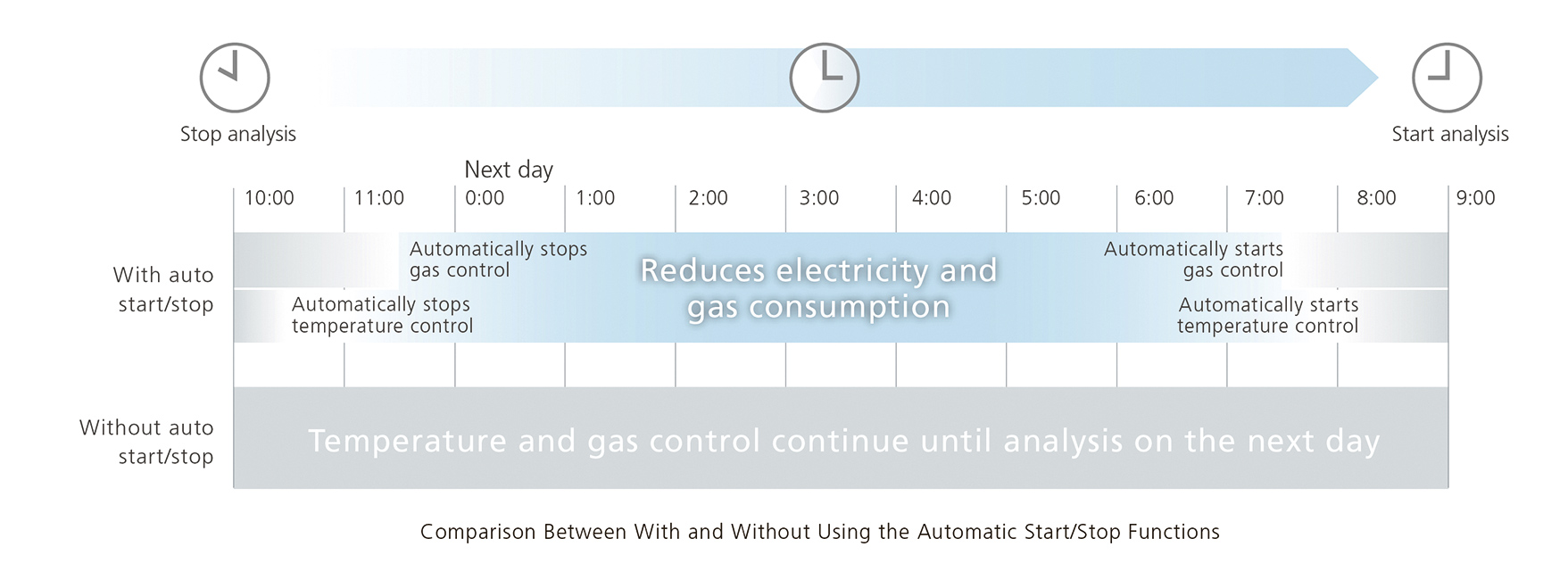
Independent of carrier gas choice, the Nexis GC-2030 provides additional measures to further decrease overall gas consumption. Carrier gas-saver functionality reduces gas flow during analysis and the gas selector can help to save helium during brief standby times. For longer standby periods, the available automatic start/stop functions reduce running costs. An automatic stop function stops GC temperature and gas control after the last analysis of a sequence, or after a sequence queue has been completed (Fig. 10).
The automatic start function can then restart the instrument from a sleeping state at any specified date and time, so that the system is up and running whenever it is needed. Automatic control samples with specific actions for an automated pass/fail evaluation can be scheduled to ensure that the instrument is operating at peak performance once the user starts work. All of this can be done automatically, leaving the operator more time for those daily tasks that really need personal supervision.
Safety. Value for money. Flexibility.
To reduce over-reliance on increasingly unavailable and progressively expensive helium, the Shimadzu Nexis GC-2030 supports cost-efficient gas chromatographic measurements with nitrogen and hydrogen as lower-cost carrier gas alternatives to helium. Nitrogen can be used as a carrier gas in conventional GC without compromising chromatographic performance and can substitute for helium as a make-up and pressurizing gas. Hydrogen allows a significant reduction of chromatographic runtimes, increasing sample throughput without compromising reliability of the results. And the Nexis GC-2030 reduces the total amount of whatever carrier gas is used.
In addition, an automatic gas selector enables convenient gas switching between different analyses and during standby periods. Smart automatic features help to optimize the GC’s daily working time and gas consumption, further reducing running costs.
Finally, the Shimadzu Nexis GC-2030 ensures lab safety for users and their equipment and offers the most economical safety option for using hydrogen as a carrier gas. The Nexis GC-2030 comes equipped with automatic leak-check functionality and hydrogen sensor solutions with automatic safety features to ensure a safe working place.
Shimadzu understands the concerns and bottom-line challenges of gas chromatography users. The Nexis GC-2030 is designed to help labs reduce costs by reducing gas-carrier amounts and by safely and flexibly utilising the full potential of lower-cost alternates to helium.