A closer look into mineral oils in food
Project develops new method for detecting DNA-reactive mineral oil fractions
Sanja Savić, Elisa Mayrhofer, Austrian Research Institute for Chemistry and Technology (OFI), Microbiology & Cell Culture
Andrea Hochegger, Erich Leitner, University of Technology Graz, Institute of Analytical Chemistry and Food Chemistry
Mineral oil contamination is common nowadays and is often in the news, from unsafe sunflower oil in 2008 and the foodwatch International report on hazardous infant formula in 2019 to the annual alerts about tainted Christmas chocolate products.[1, 2, 11] Food contaminated with mineral oil residue has long been an issue of concern and has prompted reports and risk evaluations by the European Food Safety Authority (EFSA) for many years. But how do we assess levels of contamination, and can we do it better? An Austrian government-funded research project has produced a helpful answer.




Mineral oil contamination is common nowadays and is often in the news, from unsafe sunflower oil in 2008 and the foodwatch International report on hazardous infant formula in 2019 to the annual alerts about tainted Christmas chocolate products.[1, 2, 11] Food contaminated with mineral oil residue has long been an issue of concern and has prompted reports and risk evaluations by the European Food Safety Authority (EFSA) for many years. But how do we assess levels of contamination, and can we do it better? An Austrian government-funded research project has produced a helpful answer.
Mineral oil contamination
In 2012, the European Food Safety Authority (EFSA) published a scientific opinion on the health effects of mineral oil contamination in food.[3] That report identified food packaging, different packaging additives and lubricants as the main sources of contamination. Today, it’s known that contamination can occur throughout the entire production cycle, not simply through packaging. A distinction is also made between actual food contamination and the permitted use of certain mineral oil products as food additives, e.g. microcrystalline waxes (E905). In either case, the presence of mineral oil hydrocarbons (MOH) in food means that they will be metabolized by the body during digestion.[3, 4, 5]
Separating MOSH from MOAH
MOH are complex mixtures of hydrocarbons that originate in crude mineral oil. What ends up in our food are certain subfractions, each having varying compositions of thousands of compounds. This complexity makes a clear substance-based risk evaluation difficult. During laboratory analyses, an initial separation into fractions of Mineral Oil Saturated Hydrocarbons (MOSH) and Mineral Oil Aromatic Hydrocarbons (MOAH) is possible due to their chemical structure. MOSH consist of branched and unbranched open-chain hydrocarbons and saturated cyclic hydrocarbons. Although MOSH accumulate in the human body, they are not associated with major negative effects. MOAH, on the other hand, are made up of highly alkylated aromatic substances and are considered to be potentially mutagenic and carcinogenic due to the possible presence of 3-7 ring polycyclic aromatic compounds (PACs). In crude mineral oil, this MOAH fraction makes up 15–30 %.[3, 6]
Methods in search of improvement
To ensure product quality and safety for consumers, it is important to routinely monitor, identify and eliminate MOH contamination in food. Although standardized methods are continuously improving (e.g. Update of EN16995 in 2022, JRC method for MOAH in infant formula), analysis remains challenging.[4, 15, 16] On the one hand, sample preparation is difficult, mainly due to the highly varying food matrix and the related fat content. In addition, exceptional clean-up, often manually done, is necessary, e.g. by saponification to remove fat; by Al2O3 clean-up to remove naturally occurring n-alkanes from the MOSH fraction (Alox); or by epoxidation to remove olefins from the MOAH fraction (Epox), followed by an enrichment through solvent evaporation.
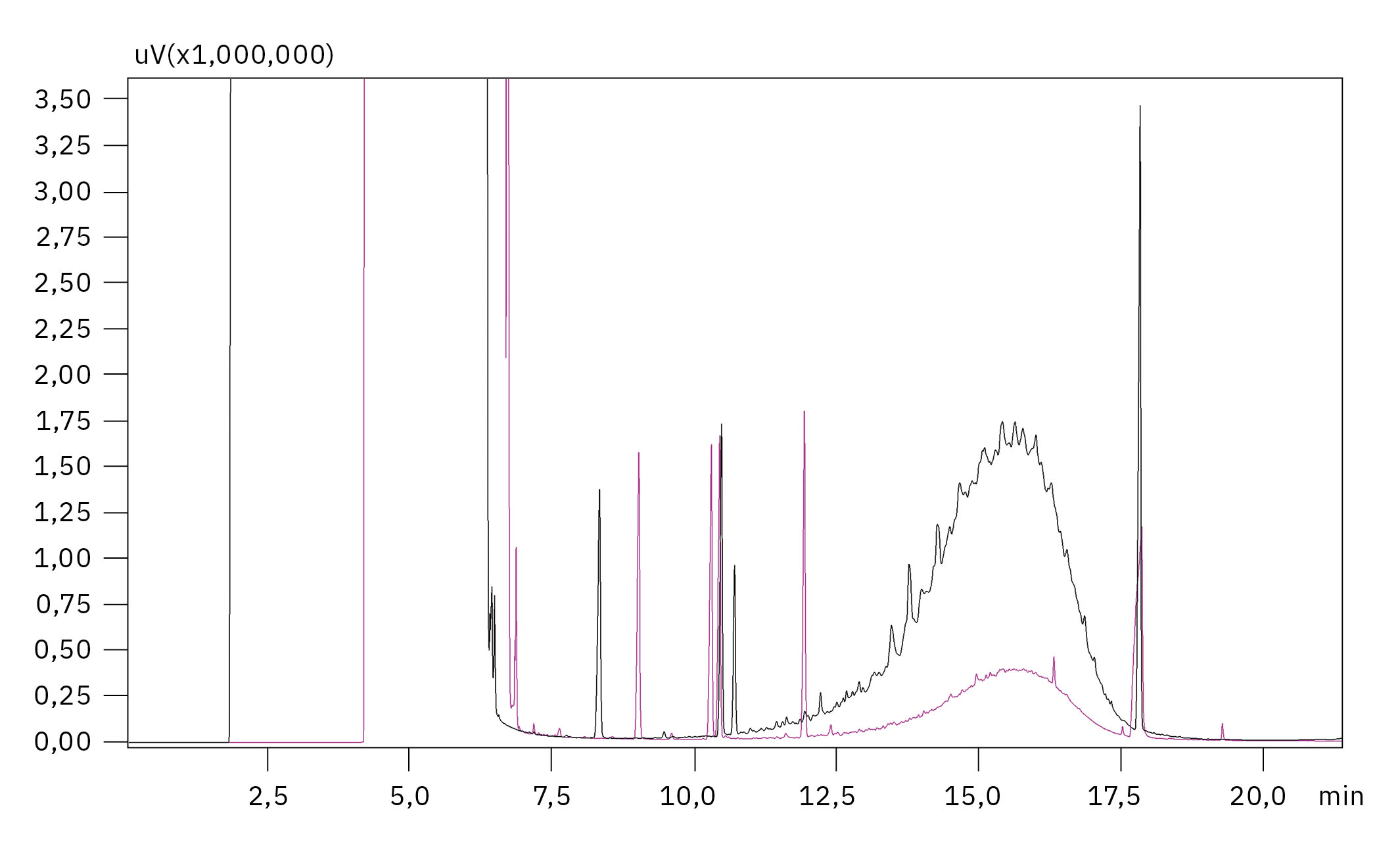
On the other hand, in state-of-the-art analysis using online-coupled HPLC-GC-FID (high-performance liquid chromatography – gas chromatography with flame ionization detection), MOH can be separated into MOSH and MOAH, but their concentration can then only be determined as a sum parameter. In this methodology, the MOH extract obtained from sample preparation is separated into the two fractions (MOSH and MOAH), using a normal phase silica column. The separated fractions are directly transferred to a GC afterwards. The GC has two separated channels (two column sets and two FIDs) to determine MOSH and MOAH in parallel in a single run. It also uses a rapid temperature program to concentrate the complex substance mixtures in large unresolved mineral-oil humps to increase the sensitivity of the method. As FIDs are universal detectors, they allow an integration of the total humps and quantification of MOSH and MOAH (see Figure 1).[7, 8] However, the many automation options available for the system offer a significant reduction in the workload of routine analysis: most of the required clean-up steps needed for state-of-art analysis according to the standardized methods, such as Alox and Epox to remove natural interferences, can be fully automated. For fats and oils, a fully automated workflow, including saponification, has also recently been introduced.[9]





Mineral oil contamination is common nowadays and is often in the news, from unsafe sunflower oil in 2008 and the foodwatch International report on hazardous infant formula in 2019 to the annual alerts about tainted Christmas chocolate products.[1, 2, 11] Food contaminated with mineral oil residue has long been an issue of concern and has prompted reports and risk evaluations by the European Food Safety Authority (EFSA) for many years. But how do we assess levels of contamination, and can we do it better? An Austrian government-funded research project has produced a helpful answer.
Mineral oil contamination
In 2012, the European Food Safety Authority (EFSA) published a scientific opinion on the health effects of mineral oil contamination in food.[3] That report identified food packaging, different packaging additives and lubricants as the main sources of contamination. Today, it’s known that contamination can occur throughout the entire production cycle, not simply through packaging. A distinction is also made between actual food contamination and the permitted use of certain mineral oil products as food additives, e.g. microcrystalline waxes (E905). In either case, the presence of mineral oil hydrocarbons (MOH) in food means that they will be metabolized by the body during digestion.[3, 4, 5]
Separating MOSH from MOAH
MOH are complex mixtures of hydrocarbons that originate in crude mineral oil. What ends up in our food are certain subfractions, each having varying compositions of thousands of compounds. This complexity makes a clear substance-based risk evaluation difficult. During laboratory analyses, an initial separation into fractions of Mineral Oil Saturated Hydrocarbons (MOSH) and Mineral Oil Aromatic Hydrocarbons (MOAH) is possible due to their chemical structure. MOSH consist of branched and unbranched open-chain hydrocarbons and saturated cyclic hydrocarbons. Although MOSH accumulate in the human body, they are not associated with major negative effects. MOAH, on the other hand, are made up of highly alkylated aromatic substances and are considered to be potentially mutagenic and carcinogenic due to the possible presence of 3-7 ring polycyclic aromatic compounds (PACs). In crude mineral oil, this MOAH fraction makes up 15–30 %.[3, 6]
Methods in search of improvement
To ensure product quality and safety for consumers, it is important to routinely monitor, identify and eliminate MOH contamination in food. Although standardized methods are continuously improving (e.g. Update of EN16995 in 2022, JRC method for MOAH in infant formula), analysis remains challenging.[4, 15, 16] On the one hand, sample preparation is difficult, mainly due to the highly varying food matrix and the related fat content. In addition, exceptional clean-up, often manually done, is necessary, e.g. by saponification to remove fat; by Al2O3 clean-up to remove naturally occurring n-alkanes from the MOSH fraction (Alox); or by epoxidation to remove olefins from the MOAH fraction (Epox), followed by an enrichment through solvent evaporation.
On the other hand, in state-of-the-art analysis using online-coupled HPLC-GC-FID (high-performance liquid chromatography – gas chromatography with flame ionization detection), MOH can be separated into MOSH and MOAH, but their concentration can then only be determined as a sum parameter. In this methodology, the MOH extract obtained from sample preparation is separated into the two fractions (MOSH and MOAH), using a normal phase silica column. The separated fractions are directly transferred to a GC afterwards. The GC has two separated channels (two column sets and two FIDs) to determine MOSH and MOAH in parallel in a single run. It also uses a rapid temperature program to concentrate the complex substance mixtures in large unresolved mineral-oil humps to increase the sensitivity of the method. As FIDs are universal detectors, they allow an integration of the total humps and quantification of MOSH and MOAH (see Figure 1).[7, 8] However, the many automation options available for the system offer a significant reduction in the workload of routine analysis: most of the required clean-up steps needed for state-of-art analysis according to the standardized methods, such as Alox and Epox to remove natural interferences, can be fully automated. For fats and oils, a fully automated workflow, including saponification, has also recently been introduced.[9]
Setting new limits
In December 2021, foodwatch International – a food-consumer-interest organization – released their findings on MOAH contamination in food. In April 2022, the Standing Committee on Plants, Animals, Food and Feed of the European Commission published a report in response to this.[10, 11] They agreed to recall products from the market across the entire European Union if they tested above a certain limit of quantification (LOQ) for MOAH. These LOQs are based on current analytical limitations of the LC-GC-FID method and depend on the fat content of the food. For food having < 4 % fat, the LOQ is 0.5 mg/kg. For food with a fat content of 4–50 %, it is set at 1 mg/kg, and for food with > 50 % fat, the limit is 2 mg MOAH/kg.[10]
It is also possible to establish limits better related to the real toxicological health effects, using a Threshold of Toxicological Concern (TTC) concept. Since MOAH is considered to be DNA-reactive, a much lower daily exposure than the LOQs is indicated: 0.15 µg/day or 0.15 µg/kg food for a person with a body weight of 60 kg.[14] This limit could be increased, but only if there were definitive proof that certain substances or sub-fractions of MOAH are not DNA-reactive. To do that, though, a more substance-based risk assessment would be needed to replace the simple determination of a sum parameter as is done by using LC-GC-FID. In 2019, EFSA recommended using additional analytical methods for samples contaminated with MOAH to identify if 3-7 ring PACs are present, as these are believed to be responsible for the DNA-reactive character of MOAH.[5] The method of choice for this characterization is 2D-comprehensive GC×GC. However, since thousands of substances may be detected with this method, risk evaluation of every single substance identified using GC×GC is not feasible. As an alternative, the entire MOAH mixture – or individual, isolated subfractions of known composition – could also be tested for their DNA-reactive effects using bioassays.
Project MOSH MOAH combines methods
To overcome the challenges inherent in current methods, a collective research project was established under the coordination of GLi Austria (Gemeinnützige Lebensmittelinitiative für Österreich) and funded by the Austrian Research Promotion Agency (FFG). During the project – “MOSH MOAH – Reduction of Mineral Oil in Food” – research partners at the University of Technology Graz (TU Graz) and the Austrian Research Institute for Chemistry and Technology (OFI) worked on developing a new assessment approach which combined state-of-the-art instrumental analysis of MOSH/MOAH with a toxicological assessment using in vitro bioassays to enable a more substance-based risk assessment of MOAH.[12]
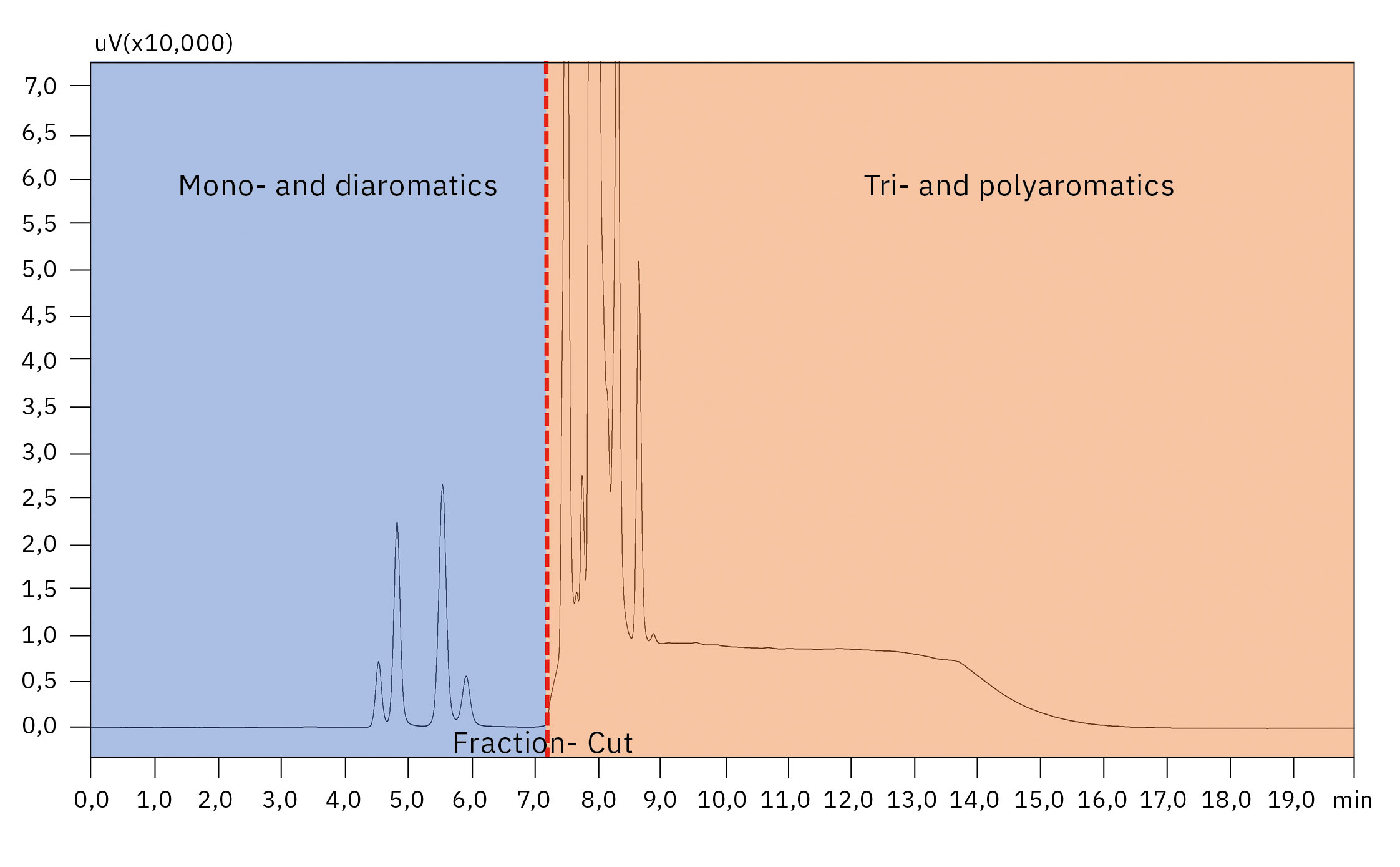
One of the main challenges facing the researchers was to separate MOAH according to ring numbers into mono- and diaromatic substances and three- and higher ring aromatics as well as to isolate those subfractions for further characterization. Koch et. al (2020) proposed a donor-acceptor complex chromatography for the group-type separation of MOAH.[13]
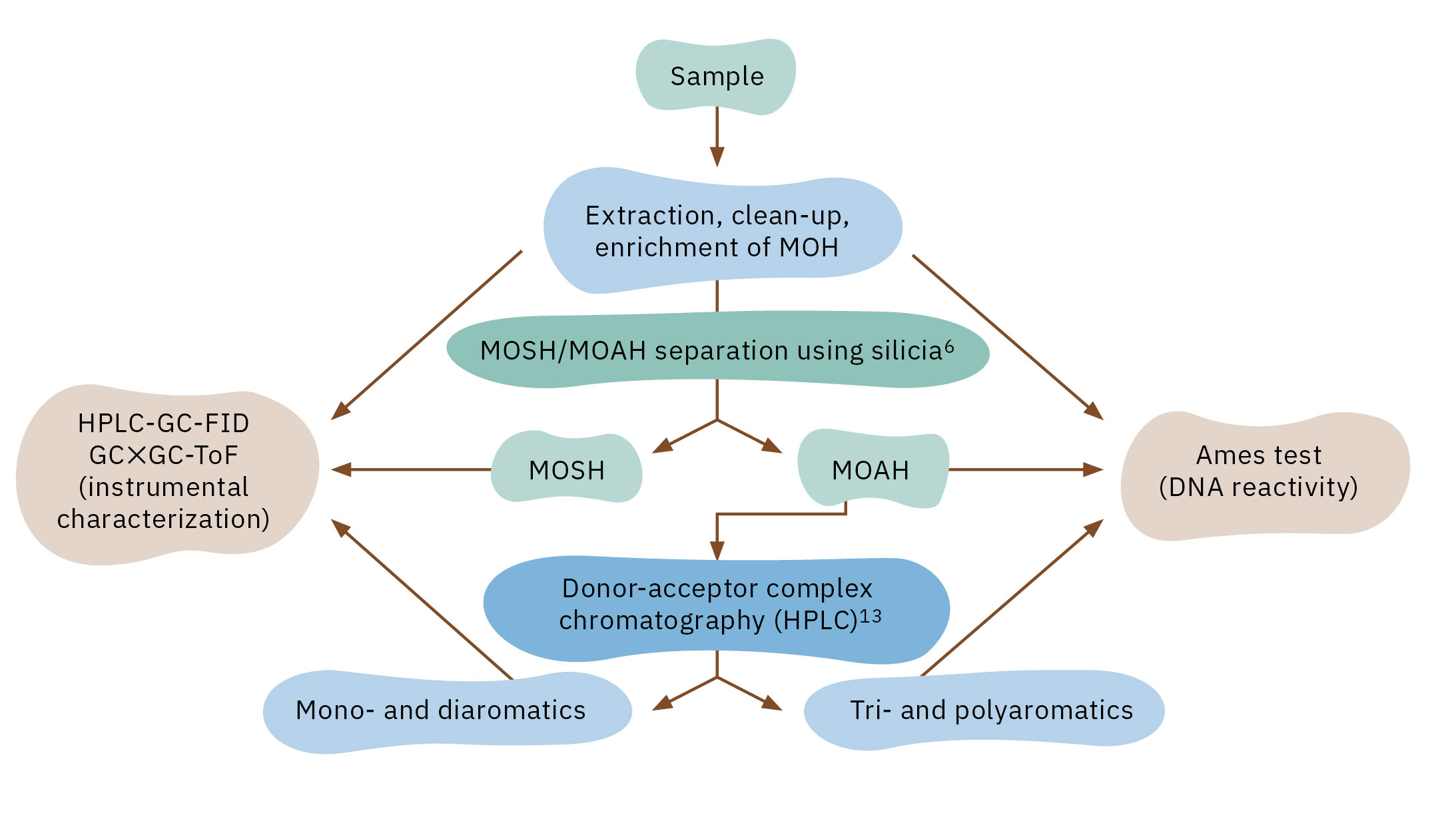
This was implemented in the project using a Shimadzu Nexera LC-20AD (Figure 2) equipped with a Nucleosil Chiral-2, 5-μm column (250 × 4 mm, MACHEREY-NAGEL GmbH & Co. KG) and a Shimadzu Prominence RF-20Axs fluorescence detector (FLD) as well as a Shimadzu SPD-M20A diode array detector (DAD). In addition, the system was equipped with a fraction collector to collect the mono- and diaromatic substances and tri- and polyaromatics isolated. The fractions were enriched and further analyzed using 2D-comprehensive GC×GC to evaluate the composition of MOAH of different origin, with a focus on the presence (or absence) of 3-7 ring PACs.
To evaluate the DNA reactivity of the isolated fractions, a miniaturized Ames test was applied. The assay used histidine auxotrophic Salmonella strains, which revert to prototrophy after contact with DNA-reactive substances, triggering growth in minimal media. Figure 3 shows the final experimental approach of the developed method that was successfully implemented in the “MOSH MOAH” project.[12]
Proof of concept
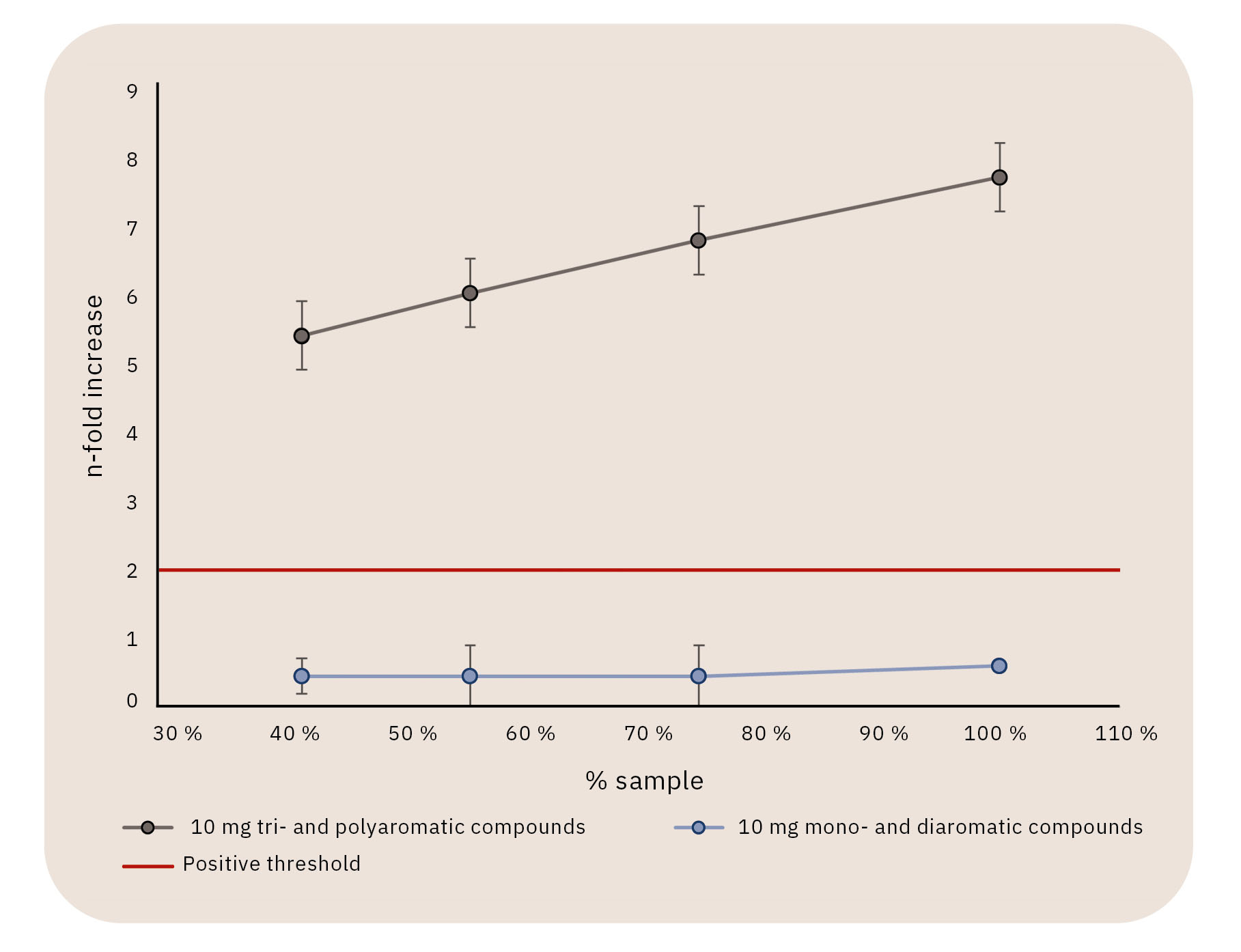
In a proof-of-concept study following the initial development of the new method, a reference mineral oil with high MOAH content was investigated.[12] The results of the instrumental characterization using GC×GC clearly showed the different composition of the isolated mono- and diaromatic fraction and tri- and polyaromatic fraction and that the separation according to the ring number had been successful. Even more interesting were the results of the bioassay: The mineral oil as such was positive in the Ames test, which meant that it showed DNA reactivity. Further, the isolated MOSH scored negative, while the isolated total MOAH was positive. A further sub-fractionation of MOAH showed that the isolated mono- and diaromatic substances were negative in the Ames test, while the tri- and polyaromatic fraction scored positive. Figure 4 shows a summary of the results.
Following the study, the developed method was applied to additional samples to collect more information on MOAH composition and DNA reactivity using different food-grade- and non-food-grade lubricants, as well as food packaging materials. The correlation observed in the proof-of-concept study was confirmed: In MOAH fractions showing DNA reactivity in the Ames test, tri- and polyaromatic substances could be detected using GC×GC, while they were absent in non-DNA-reactive MOAH fractions. The conclusion is gratifying: Using this combined approach – and the knowledge it generates – offers a clearly better way to identify hazards and assess risks in food samples contaminated with MOAH.
Dedication to purity
In the “MOSH MOAH – Reduction of Mineral Oil in Food” project, researchers were able to actively contribute to overcoming a significant challenge in the monitoring of mineral oil residue in food. Existing methods were improved upon and new strategies developed to make identification of contamination sources easier as well as to reduce contamination and to assess the related risk using a more substance-based approach.
The knowledge generated by this project will be particularly useful to the food packaging and food industries in their constant quest to improve the quality of food and ensure consumer safety by reducing mineral oil contamination. Shimadzu is proud that its precision instruments could be of use to the dedicated scientists who are helping us all.

[1] Biedermann M, Grob K. Comprehensive two-dimensional GC after HPLC preseparation for the characterization of aromatic hydrocarbons of mineral oil origin in contaminated sunflower oil. J Sep Sci. 2009; https://doi.org/10.1002/jssc.200900366
[2] foodwatch. Project-report: International test of various canned baby milk products for their content of mineral oil hydrocarbons (MOSH/MOAH): A project of foodwatch International with foodwatch Germany, foodwatch Netherlands and foodwatch France; 2019.
[3] EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF). Scientific Opinion on Mineral Oil Hydrocarbons in Food. EFSA (EFSA Journal). 2012; https://doi.org/10.2903/j.efsa.2012.2704
[4] Hochegger A, Moret S, Geurts L, Gude T, Leitner E, Mertens B, O’Hagan S, Poças F, Simat TJ, Purcaro G. Mineral oil risk assessment: Knowledge gaps and roadmap. Outcome of a multi-stakeholders workshop. Trends in Food Science & Technology. 2021; https://doi.org/10.1016/j.tifs.2021.03.021
[5] Arcella D, Baert K, Binaglia M. Rapid risk assessment on the possible risk for public health due to the contamination of infant formula and follow‐on formula by mineral oil aromatic hydrocarbons (MOAH). EFSA. 2019; https://doi.org/10.2903/sp.efsa.2019.en-1741
[6] Fiselier K, Grundböck F, Schön K, Kappenstein O, Pfaff K, Hutzler C, Luch A, Grob K. Development of a manual method for the determination of mineral oil in foods and paperboard. J Chromatogr A. 2013; https://doi.org/10.1016/j.chroma.2012.11.034
[7] Biedermann M, Grob K. On-line coupled high performance liquid chromatography-gas chromatography for the analysis of contamination by mineral oil. Part 1: method of analysis. J Chromatogr A. 2012; https://doi.org/10.1016/j.chroma.2012.05.095
[8] Biedermann M, Grob K. On-line coupled high performance liquid chromatography-gas chromatography for the analysis of contamination by mineral oil. Part 2: migration from paperboard into dry foods: interpretation of chromatograms. J Chromatogr A. 2012; https://doi.org/10.1016/j.chroma.2012.05.096
[9] Nestola M. Automated workflow utilizing saponification and improved epoxidation for the sensitive determination of mineral oil saturated and aromatic hydrocarbons in edible oils and fats. Journal of Chromatography A;2022; https://doi.org/10.1016/j.chroma.2022.463523.
[10] Standing Committee on Plants, Animals, Food and Feed. SUMMARY REPORT: Mineral oil hydrocarbons in food: follow-up to the December 2021 Foodwatch report. 2022.
[11] foodwatch International. Toxic mineral oil in food – Laboratory tests 2021;2021. https://www.foodwatch.org/fileadmin/-INT/mineral_oil/documents/Foodwatch_Mineralo__l_Report_2021_ENGLISH_03A.pdf [last accessed 05.03.2023]
[12] Hochegger A, Wagenhofer R, Savić S, Mayrhofer E, Washüttl M, Leitner E. Combination of Multidimensional Instrumental Analysis and the Ames Test for the Toxicological Evaluation of Mineral Oil Aromatic Hydrocarbons. J Agric Food Chem. 2022; https://doi.org/10.1021/acs.jafc.2c05970
[13] Koch M, Becker E, Päch M, Kühn S, Kirchhoff E. Separation of the mineral oil aromatic hydrocarbons of three and more aromatic rings from those of one or two aromatic rings. J Sep Sci. 2020; https://doi.org/10.1002/jssc.201900833
[14] More, Simon J.; Bampidis, Vasileios; Benford, Diane; Bragard, Claude; Halldorsson, Thorhallur I.; Hernández-Jerez, Antonio F. et al. (2019): Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. In: EFS2 17 (6), e05708. https://doi.org/10.2903/j.efsa.2019.5708
[15] Bratinova S., Robouch P., Beldi G., Senaldi C., Karasek L., Gonçalves C,. Valzacchi S., Garcia-Ruiz S., Hoekstra E. (2023): Determination of MOAH in Infant Formula. JRC IF 2022-05 : the ring trial validation study. Hg. v. Publications Office of the European Union. Joint Research Centre (European Commission). Luxembourg. Online verfügbar unter https://op.europa.eu/en/publication-detail/-/publication/3220b5f8-d9ac-11ed-a05c-01aa75ed71a1/language-en, zuletzt geprüft am 10.05.2023.
[16] DGF method C-VI 22 (20) “Mineral oil constituents, MOSH and MOAH with online coupled LC-GC-FID method for low limits of determination and Draft version for updating EN-16995:2017, September 2022-V4.