Bisquaternary ammonium adduct with trifluoroacetate
MSn fragmentation analysis of bisquaternary ammonium-trifluoroacetate adduct
Marta Kowalska, Dr. Remigiusz Bąchor, Faculty of Chemistry, University of Wroclaw
Trifluoroacetate (TFA) is a mobile and persistent substance that is primarily introduced into the water cycle through the degradation of various fluorochemicals and remains in the environment in the long term. Also known as an ion-pairing agent, it reduces the signal intensity in the ESI-MS spectrum. Nonetheless, the examination of its binding by mass spectrometry has the potential to have a significant research impact on the formation of appropriate sensors.
In this study, we demonstrate the feasibility of identifying a stable bisquaternary adduct with trifluoroacetate, resulting in the formation of distinctive fragment ions in MSn mode containing a covalently bound anion. This phenomenon requires bond reorganization, which leads to charge retention on the nitrogen atom.
The formation of adducts is a common occurrence in electrospray ionization-mass spectrometry (ESI-MS) analysis.[1] One common observation is the presence of potassium, sodium, ammonium or lithium adducts in the positive ion mode [2] and chlorine adducts in the negative ion mode.[3, 4] Trifluoroacetic acid may form adducts with positively charged analyte ions in the positive ion mode of ESI-MS, which reduces the signal intensity on the mass spectrum. This phenomenon is common due to the widespread use of TFA as an additive in high-performance liquid chromatography (HPLC). The utilization of TFA results in an enhancement of the chromatographic peak shape owing to the reduction of the silanol-group effect from columns.[5] For mass spectrometry analysis, it is important to understand the interaction between two associated molecules from a mechanistic and biological perspective. Certain compounds exhibit a low ionization efficiency during the ESI-MS experiment, thereby limiting their reliable identification. Moreover, as previously mentioned, the presence of anions, such as the TFA anion, may result in a decrease in signal intensity on the MS spectrum, which may pose a challenge during the analysis of certain compounds that are hardly ionized. The use of fixed charge tags, such as quaternary ammonium salts, is one of the approaches, which enhances the effectiveness of ionization and detection sensitivity.[6] Quaternary ammonium cations are nitrogen-containing ions with four aryl or alkyl chains attached to the nitrogen (NR4+ structure). Consequently, quaternary ammonium cations possess a constant positive charge.[7] The application of quaternary ammonium salts allows reducing the limitations of the ESI-MS/MS analysis due to the introduction of positive charge, thereby enabling the analysis of compounds with low ionization efficiency.[6]
Pyridinium group enhances ionization efficiency
Pyrylium salts are an example of a compound that has a constant positive charge due to the presence of a positively charged oxygen atom. The high reactivity of this atom toward nucleophiles has led to the use of pyrylium salts in the creation of pyridinium derivatives, compounds containing the quaternary ammonium atom. The presence of the pyridinium group, owing to its constant positive charge, enhances the efficiency of ionization, resulting in enhanced sensitivity, such as in the detection of peptides.[8]
In the presented research, the gas phase formation of a non-covalent bisquaternary ammonium-trifluoroacetate adduct was described, even during an MS/MS experiment and monitored by Shimadzu LCMS-IT-TOF (Shimadzu, Kyoto, Japan).
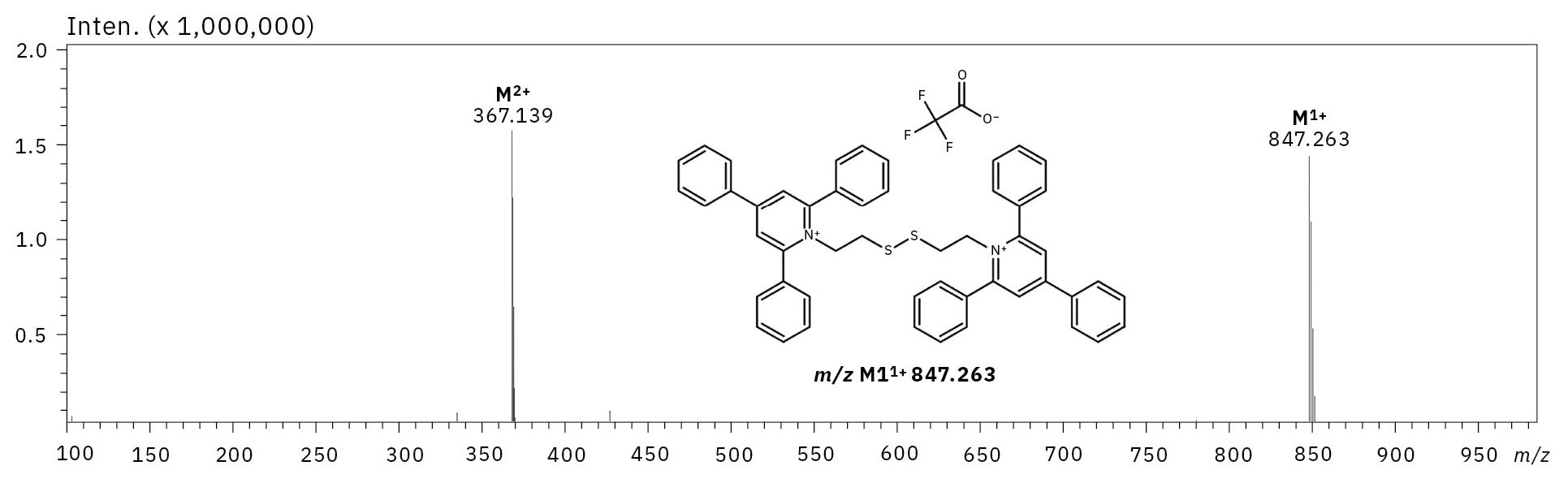
The model bisquaternary ammonium compound in the form of 2,2’-disulfanediylbis(2,4,6-triphenylpyridinium)((TPP)2-CYSTAM) was synthesized through the reaction between 2,4,6-triphenylpyrylium tetrafluoroborate and cystamine in the presence of N,N,N-triethylamine. The synthesized compound was purified using the reverse-phase HPLC, where the trifluoroacetic acid was used as mobile phase additive. The purified compound was then analyzed by ESI-MS (Figure 1). The analysis of (TPP)2-CYSTAM mass spectrometry in positive ion mode revealed the presence of the signal at m/z 367.139, which corresponds to the M2+ ion. The ESI-MS and ESI-MS/MS analysis confirmed the success of the synthesis.
Additionally, the ESI-MS spectrum (Figure 1) revealed the presence of the signal at m/z 847.263 (M11+). The isotopic pattern of the signal at m/z 847.263 (M11+) was practically the same as in the case of the signal at m/z 367.139 (M2+). The detailed analysis showed that the signal at m/z 847.263 characterizes the +1 ion, which contains two sulfur atoms. The mass of the formed ion was found to be 113 Da higher than the mass of the bisquaternary ammonium compound (M2+ ion), which is characteristic to trifluoroacetate (112.986 Da). As it was mentioned above, TFA used in the mobile phase can cause suppression effects and decrease signal intensity.[5] However, the (TPP)2-CYSTAM has two positive charges, and during the formation of the (TPP)2-CYSTAM-TFA adduct only one positive charge is neutralized. Therefore, the formation of a singly charged non-covalent adduct is observed, which is stable in the gas phase. To check the stability of the (TPP)2-CYSTAM-TFA adduct, the ESI-MS/MS analysis was performed (Figure 2).


Performing the fragmentation of parent ion
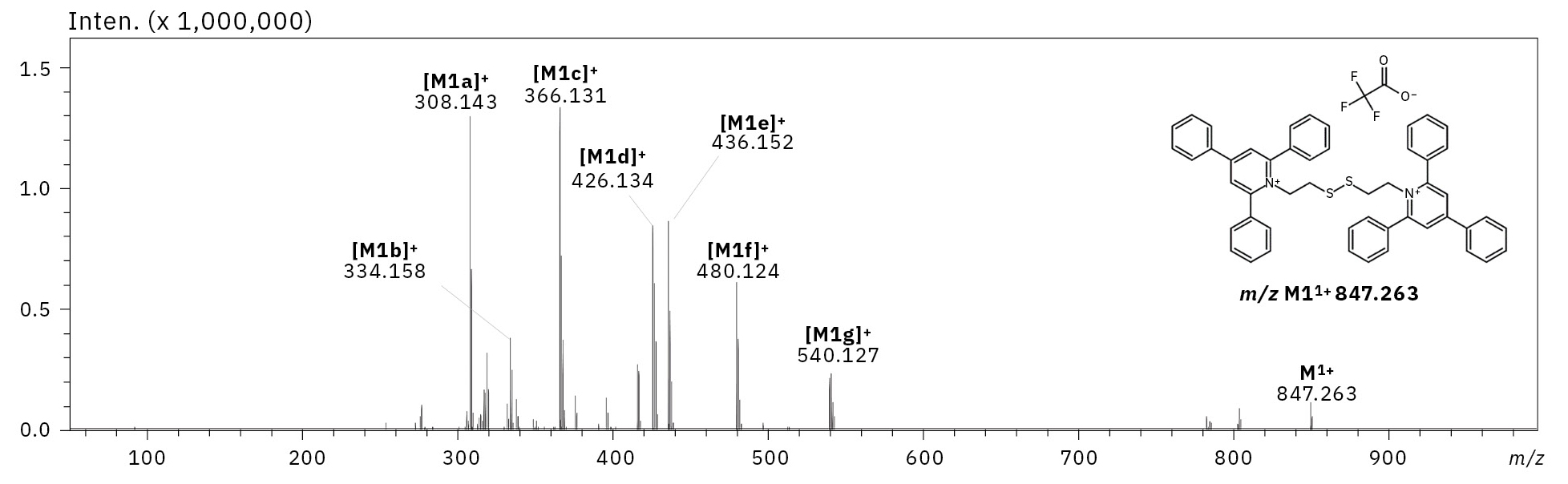
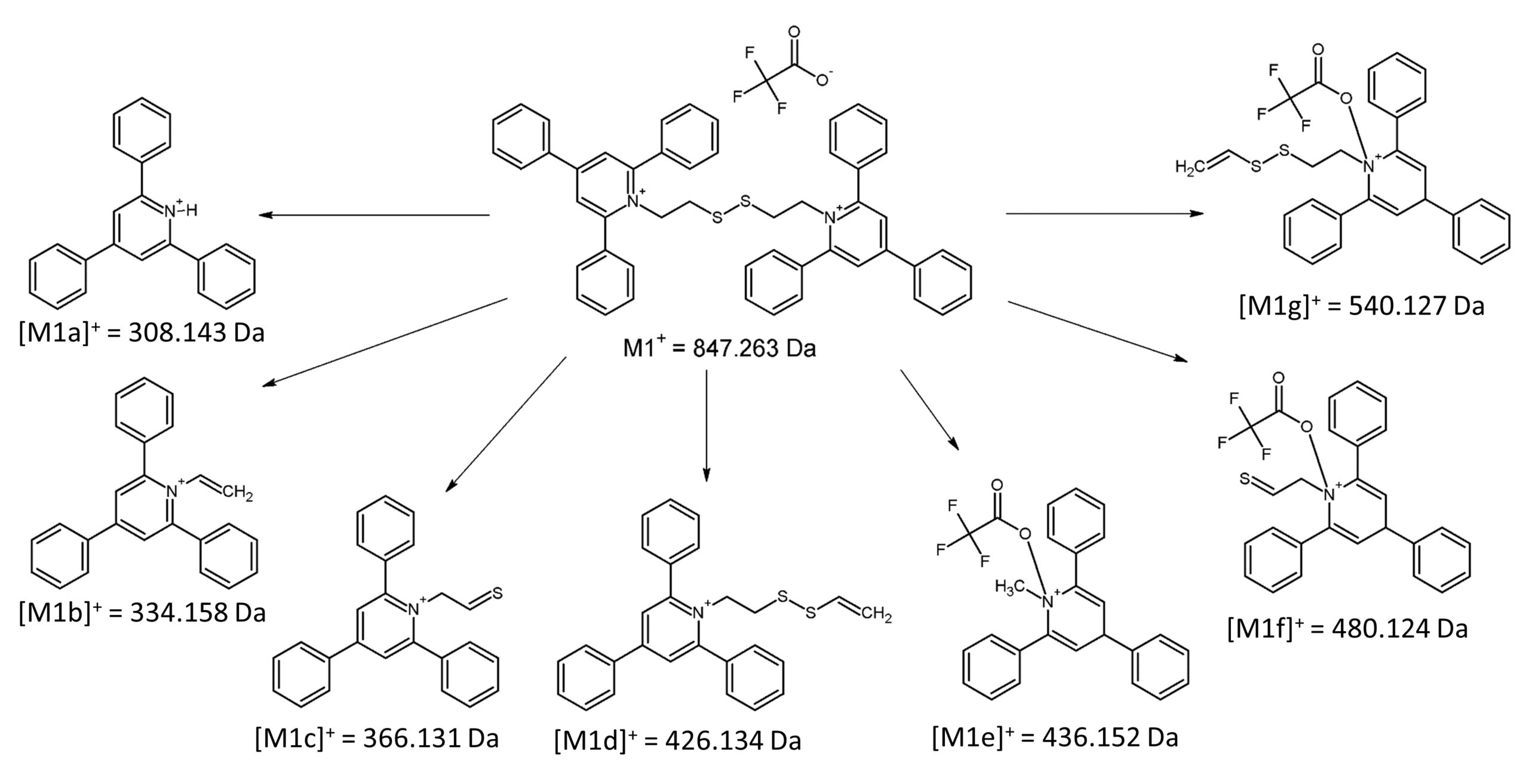
In general, the adduct formed in ESI-MS does not show any other fragmentation than cation or anion loss in ESI-MSn experiments. Therefore, the fragmentation of the parent ion at m/z 847.263 (M1+) was performed. One of the most characteristic fragment ions was the [M1a]+ ion at m/z 308.143, which corresponds to the protonated form of 2,4,6-triphenylpyridine (TPP). TPP is a commonly known reporter ion, which is formed during fragmentation of the TPP-modified peptides.[8] Apart from the [M1a]+ ion, the fragmentation revealed the formation of the fragment ions [M1b]+, [M1c]+, [M1d]+ at m/z respectively 334.158, 366.131 and 426.134, which were characteristic to dissociation of fragments of bisquaternary ammonium compound (Figure 2, 3).
If the formed adduct is stable in the gas phase, the part of the molecule that remains with a positively charged quaternary nitrogen atom after dissociation should be neutralized by the presence of the trifluoroacetate anion. However, the obtained mass spectrum presents other signals that correspond to the fragment ions, which mass do not indicate the loss of the TFA moiety. The detailed analysis revealed that the signals characterizing ions at m/z 436.152 [M1e]+, 480.124 [M1f]+ and 540.127 [M1g]+ are the TFA adducts with a singly charged nitrogen atom.
It is not straightforward to identify signals characterizing positively charged ions in the form of trifluoroacetate adducts, as it would require either additional charge formation or reorganization of chemical bonds. Furthermore, the m/z values of the signals depicted in the obtained ESI-MS/MS spectrum for fragment ions corresponding to the TFA adducts are shifted by 1 Da in comparison to the non-covalent form of the TFA adduct, which may indicate the occurrence of bond reorganization. The schematic presentation of the formed ions and corresponding m/z values are presented in Figure 3. Moreover, ESI-MS3 analysis of a parent ion [M1g]+ at m/z 540.127 revealed the formation of a fragment ion at m/z 480.124, which also corresponds to the TFA adduct ([M1f]+).
Laying the foundation for a future anion sensor for environmental samples
To summarize, we have demonstrated the formation of a stable bisquaternary ammonium-trifluoroacetate adduct in the gas phase during ESI-MS experiments, which does not result in the ion suppression. The MS/MS analysis showed that electrostatic interactions between bisquaternary ammonium compound and trifluoroacetate moiety can be converted into covalent bonds in the presence of collision energy. TFA anion forms the stable N-O bond with the bisquaternary ammonium cation (confirmed by computational methods based on DFT), which increase stability of the obtained adduct. The conversion to the covalent system requires bond reorganization and charge retention on the nitrogen atom. This occurrence makes it possible to observe the fragment ions containing the TFA moiety. The aforementioned interactions and the potential for their analysis in the future may result in the development of an anion sensor that can be utilized to determine the presence of certain anionic compounds in environmental samples. The results of the conducted research significantly enhance the potential of mass spectrometry in identifying interactions between cations and anions in the gas phase. This enables the utilization of this technique in identifying specific anions in samples.
The research project is supported/partly supported by the program „Excellence initiative – research university”, lasting from 2020 until 2026.
The author would like to thank Andrzej Reszka (Shim-Pol, Poland) for providing access to the Shimadzu LCMS-IT-TOF instrument.
[1] Kruve, A., Kaupmees, K. (2017).J A So Mass Spectrom. 28: 887–894.
[2] Steckel, A., Schlosser, G. (2019). Molecules. 24: 611.
[3] Kuksis, A., Marai, L., Myher, J.J. (1991). J Chromatogr A. 588: 73–87.
[4] Kuksis, A., Marai, L., Myher, J.J. (1991). Lipids. 26: 240–246.
[5] Wouters, S., Eeltink, S., Haselberg, R., Somsen, G.W., Gargano, A.F.G. (2021). Anal Bioanal Chem. 413: 4379–4386.
[6] Bąchor, R., Mielczarek, P., Rudowska, M., Silberring, J., Szewczuk, Z. (2014). Int J Mass Spectrom. 362: 32–38.
[7] Shackman, H.M., Ding, W., Bolgar, M.S. (2015). J Am Soc Mass Spectrom. 26: 181–189.
[8] Waliczek, M., Bąchor, R., Kijewska, M., Panek-Laszczyńska, K., Konieczny, A., Dąbrowska, K., Witkiewicz, W., Marek-Bukowiec, K., Tracz, J., Łuczak, M., Szewczuk, Z, Stefanowicz, P. (2019). Anal Chim Acta. 1048: 96–104.