Individually dosed
Therapeutic drug monitoring with LC/MS/MS

Therapeutic drug monitoring (TDM) generally refers to determination of specific drug concentrations in blood at specified time intervals. The purpose of TDM is to secure a relatively constant concentration, the so-called steady state plasma concentration, of the corresponding drug in the bloodstream. Many fields of medicine use this method in daily clinical practice to obtain the optimal dosage of drugs with a relatively narrow therapeutic index.
For drugs with a narrow therapeutic index, the effective blood concentration required may be close to concentrations which are already toxic or cause, at least, undesirable side-effects. At the same time, maintaining the therapeutically effective concentration (uptitration) for certain drugs is not as easy as administering a standard dose. Each person absorbs, metabolizes, utilizes and eliminates drugs differently. Factors such as age, gender, general state of health, genetic makeup or interferences with other drugs all play a role.
Life changes – and with it medication
Many drugs that are therapeutically monitored are administered throughout life. And just as life holds many changes with new situations, the drug dosage administered may also need to be adjusted from time to time. The effects of changed life circumstances and, possibly, the altered pharmacokinetics of the drug can be controlled via TDM and the drug dosages can be adjusted accordingly.
Substances determined by TDM include analgesics, antiarrhythmics, antibiotics, antidepressants, antiepileptics, immunosuppressants and cytostatics. In the context of therapeutic drug monitoring, prompt availability of the required drug level, for many critical patients highly important, should be assured. Fast and reliable analytical methods play an important role here.
At the same time, analytical methods for quantitative detection of the various drugs to be monitored must be sufficiently sensitive and selective. Immunological methods offer a fast and straightforward solution, as it is often not necessary to separate the drug from the matrix (blood, plasma, serum etc.). A major disadvantage of immunological methods is, however, the very high susceptibility to interfering substances, which under certain circumstances can lead to false results, due for instance to cross-reactivity of metabolites of the active substance. The concentrations of active substances and active metabolites are often expressed as the sum of both.
Highly selective analysis method: multi-reaction monitoring
The use of liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS) offers an excellent alternative to immunological methods. The underlying methodology of ‘multi-reaction monitoring’ (selecting the desired parent substance in the first quadrupole [Q1], fragmenting this substance in the collision cell [Q2], detecting one or more specific fragments in the third quadrupole [Q3]) (Fig. 1: Schematic representation of the triple quadrupole) makes this analytical method superior to other methods in terms of selectivity. It is often possible to analyze many compounds using the same method, even without time-consuming sample preparation steps.
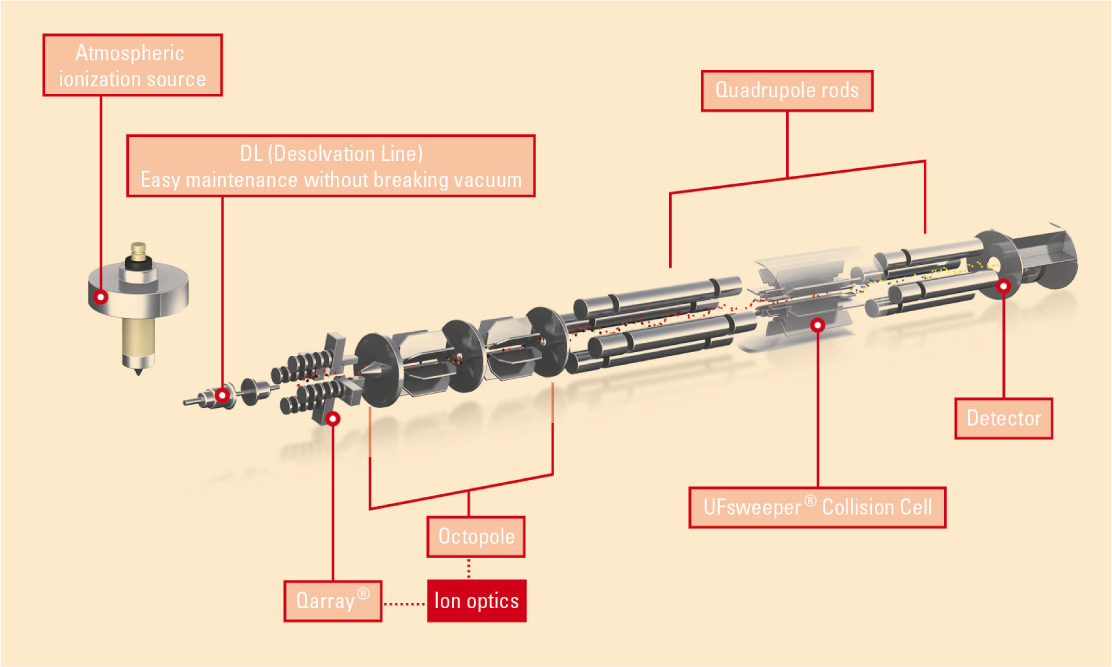 Figure 1: Schematic representation of the triple quadrupole
Figure 1: Schematic representation of the triple quadrupole
LC/MS/MS user kits for routine diagnostics
Shimadzu offers with the LCMS-8030 a triple quadrupole mass spectrometer (Figure 3) meeting all of today’s requirements in therapeutic drug monitoring. Since TDM is now part of routine clinical diagnostics, various manufacturers offer complete user kits for the analysis of, for example, immunosuppressants, neuroleptics, antidepressants, benzodiazepines etc. Time-consuming method development and validation is no longer needed when using these types of kits, and even relatively inexperienced users can set up reliable analysis methods for routine diagnostics using LC/MS/MS.
 Figure 3: The LCMS-8030 is one of the fastest systems on the market with a polarity switching time of just 15 msec, a scan speed of 15,000 u/sec and dwell times of 1 msec
Figure 3: The LCMS-8030 is one of the fastest systems on the market with a polarity switching time of just 15 msec, a scan speed of 15,000 u/sec and dwell times of 1 msec
A kit usually includes calibrator sets as well as control samples containing the substances to be analyzed, the required mobile phases and rinsing solutions for HPLC, and an analytical column (depending on the kit, also a trapping column or SPE [solid phase extraction] column) as well as all necessary solutions for sample preparation, which often consists of a less time-consuming protein precipitation. Furthermore, an internal standard is usually added to the samples – ideally these are deuterated standards of the actual analyte or analogues with similar chemical characteristics. They are commonly added to the standards in order to compensate for possible extraction losses or influences that can distort the analysis results.
When speed counts …
Of course, Shimadzu’s LCMS-8030, as well as the manufacturers of analytical kit systems takes fast analysis times into account. It is, for instance, possible to determine 33 benzodiazepines resp. their metabolites together with 20 internal standards (Figure 2, sample chromatogram) in only eleven minutes or to determine the four most common immunosuppressants (sirolimus, tacrolimus, everolimus and cyclosporine A) in 4.5 minutes – or even 1.3 minutes for those who are in a hurry. Fast quantitative analysis can then be carried out using Shimadzu’s flexible and user-friendly LabSolution software.
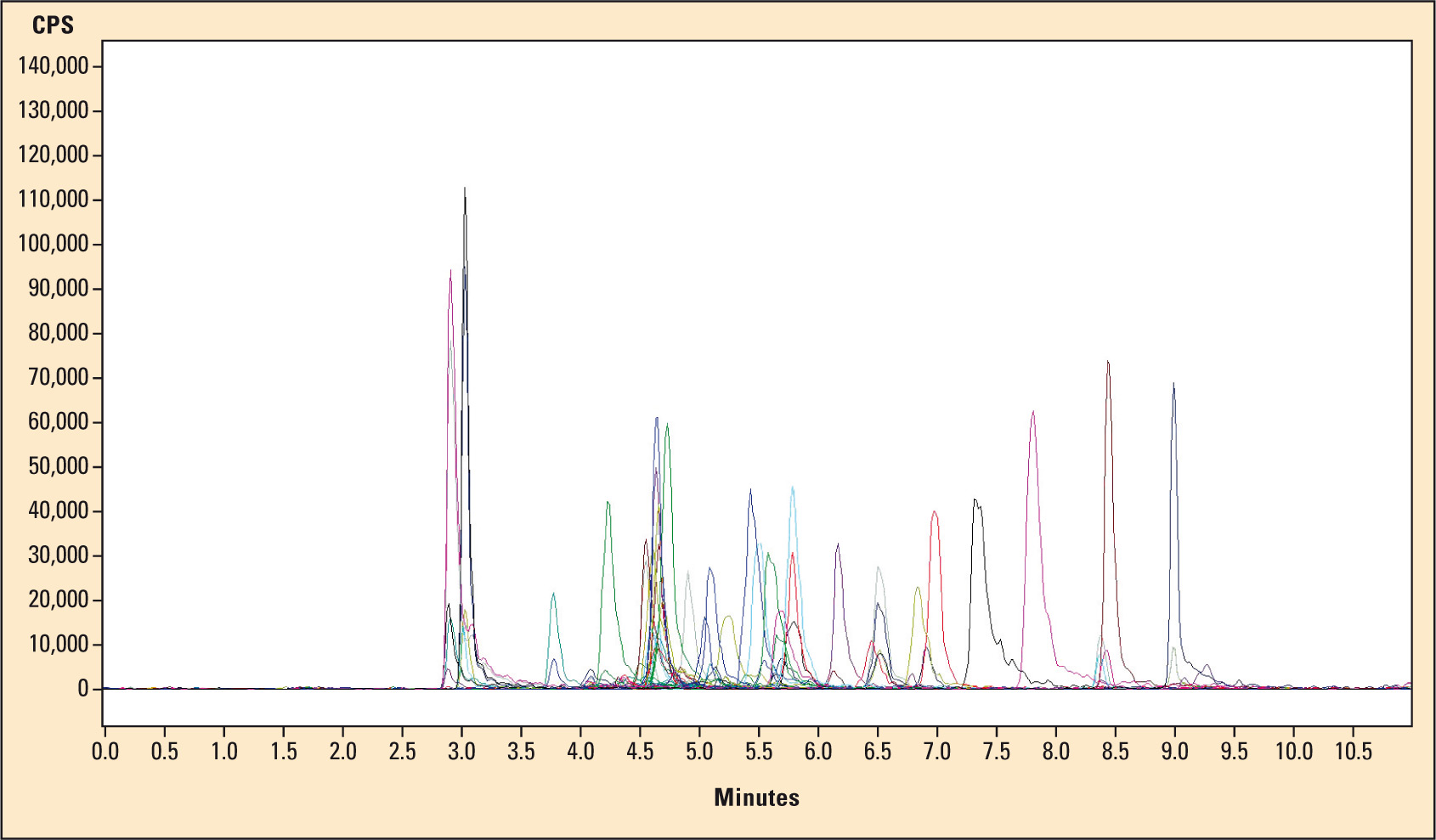 Figure 2: Example chromatogram of an analysis of 33 benzodiazepines respectively their metabolites using 20 internal standards
Figure 2: Example chromatogram of an analysis of 33 benzodiazepines respectively their metabolites using 20 internal standards