Vanillin – natural or synthetic?
GCMS and IRMS acquire the isotopic fingerprint of vanillin
 Natural vanilla. The name comes from the Spanish »vainilla«, small pod.
Natural vanilla. The name comes from the Spanish »vainilla«, small pod.
Vanillin is the most popular flavor worldwide. It is traditionally produced from vanilla pods (Vanilla planifolia or other species) by maceration. However, natural vanilla production covers only 1 % of global demand. 99 % of vanillin flavor is produced synthetically (petrochemical origin) or biotechnologically (e.g. from ferulic acid, eugenol).
Between natural and synthetic vanillin is a cost factor of 100:1. It’s no wonder that vanillin is one of the most imitated additives in food products worldwide. How can consumers be protected from false declaration and fraud?
Natural and synthetic vanillin is chemically identical, but an isotopic signature is left by the raw material and the production process. Consequently, the “isotopic fingerprint” can be used for differentiation.
GCMS and IRMS reveal compound-specific isotope signature
A common elemental analyzer, coupled to a high resolution sector field mass spectrometer (Isotope Ratio Mass Spectrometer, IRMS) can be used for the determination of the isotope signature from pure material with high precision and accuracy. However, the vanillin (target compound) in a vanilla extract from a complex matrix ranges in concentration from 2-300 µg mL-1.
The substances should therefore first be separated from each other, after which isotopic ratios of the target compounds as well as of accompanying compounds when needed can be determined (Compound Specific Isotope Analysis, CSIA). This can be accomplished by coupling of GCMS and IRMS. The Austrian-based company Imprint Analytics uses a Shimadzu GCMS (GCMS-QP2010 Ultra) for this coupling technique as a worldwide first.
The CSIA technique was first used commercially for the determination of the 13C/12C ratio in vanillin (Hoffman und Salb, 1979 [1]). However, the determination of only 13C cannot differentiate between synthetic (petrochemical) and biotechnological (e.g. ferulic acid, eugenol) origin of vanillin. Furthermore, the 13C signature can be manipulated by enriched raw material so as to counterfeit a natural product. More differentiation characteristics should be included.
Determination of the isotopic ratios of hydrogen
For the above reason, more differentiating parameters should be determined. Additional determination of the isotopic ratios of hydrogen has been proposed (Greule et al., 2010 [2]). However, little data is available for this today. Imprint Analytics has built up a large database in the past years which can be used to determine the origin and authenticity of such samples.
Which methods does Imprint Analytics use to verify whether vanillin is from natural, nature-identical or synthetic origin in extracts and end-products?
Materials and methods
Vanillin is extracted from an essence (or from the end-product). The solvent is 2-Methoxy-2-methylpropane (MTBE). 200 µL of the sample are used on the auto sampler (Shimadzu, AOC-5000).
Two separate analyses are performed to determine the isotopic signatures of carbon and hydrogen.
The samples are cold injected with a 10 µL syringe into the Optic-4 Multimode Inlet (GL Sciences, Tokyo, Japan) at 35 °C. The solvent is removed for some seconds by blowing it out and the injector is then heated up to 285 °C. The sample is transferred onto the column by splitless injection mode. Separation is done using a ZB-5MSi column (60 m x 0.25 mm 1.4 µm, Phenomenex, Torrance, USA). The GC program is:
Temperature program: 35 °C, 1 min,
Heating rate: 20 °C min-1 until 300 °C,
Pressure program: He carrier: 300 kPa, flow control: linear velocity, APC1 (pressure after Detector Switching Device, DSD) 100 kPa; Splitless time: 0.7 min.
The DSD from Shimadzu is located after the separation column. It enables total separation of the solvent and transports other substances into the heart-cut purge. Vanillin and other substances with similar retention times are transported by a T-piece into an inert silica glass capillary with two directions:
- separate substances which are identified in the Shimadzu GCMS-QP2010 Ultra
- organic substances which are oxidized in the oven into H2O und CO2 (Hekatech, Wegberg, Germany).
Water is removed with a NafionTM membrane from the He flow (Hekatech, Wegberg, Germany) and CO2 gas is forwarded to the IRMS (Nu Instruments, Wrexham, UK) where the isotopic ratio 13C/12C is determined (figure 1).
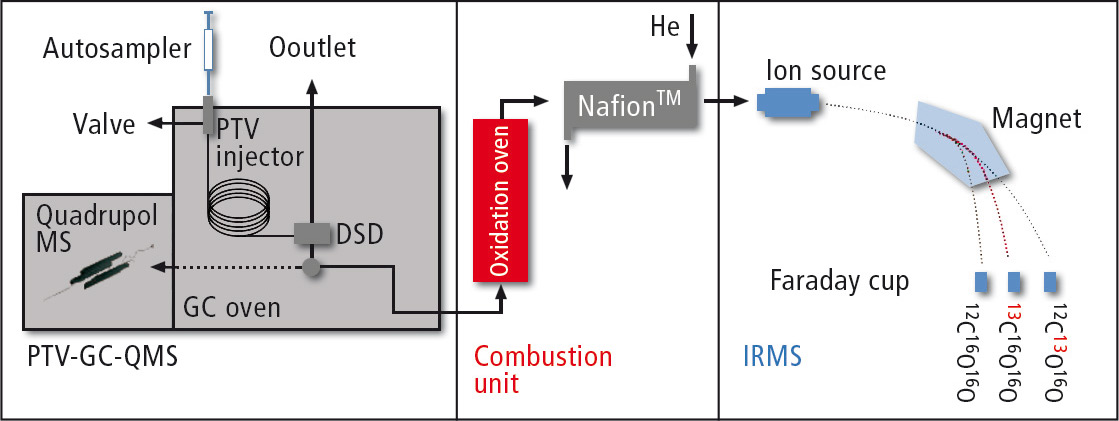 Figure 1: Method for measuring the carbon isotopic ratio in vanillin (schematic drawing). The sample is cold injected and the solvent is blown out from the PTV (vent).The Detector Switching Device (DSD) from Shimadzu is located after the separation column, where the matrix substances are separated. The gas flow is split after the DSD: the smaller part is transferred to the quadrupole mass spectrometer, where the substances are identified; the larger part is flashed in the oxidation oven. The CO2 is transferred into the IRMS after removal of water and the isotopic ratio is measured.
Figure 1: Method for measuring the carbon isotopic ratio in vanillin (schematic drawing). The sample is cold injected and the solvent is blown out from the PTV (vent).The Detector Switching Device (DSD) from Shimadzu is located after the separation column, where the matrix substances are separated. The gas flow is split after the DSD: the smaller part is transferred to the quadrupole mass spectrometer, where the substances are identified; the larger part is flashed in the oxidation oven. The CO2 is transferred into the IRMS after removal of water and the isotopic ratio is measured.
The determination of hydrogen isotopes is similar, but more sample material is required. Injection volume and blow-out time in the Optic-4 Multimode Inlet are therefore increased. Vanillin is carried into the pyrolysis oven instead of an oxidation oven. In the pyrolysis oven, vanillin is reduced to H2 (and CO). H2 is transferred to the IRMS to determine the isotopic ratio of 2H/1H.
Measured values are normalized in two steps to ensure high measurement accuracy: each run with a reference gas, and each sample row with a reference material (in case of hydrogen two reference materials) with known isotopic ratio. The normalization process is verified by quality control samples. More replicate measurements are carried out for both elements to ensure a high precision.
Measured values are compared with a reference database (figure 2). In this way, it is determined whether vanillin is from natural, nature-identical, or synthetic origin. The composition of the isotopes can also be used to prove authenticity and the determination of the geographical origin of the natural vanillin. The declaration of bourbon vanilla can be verified by this procedure, e.g. whether the vanilla really originates from Madagascar.
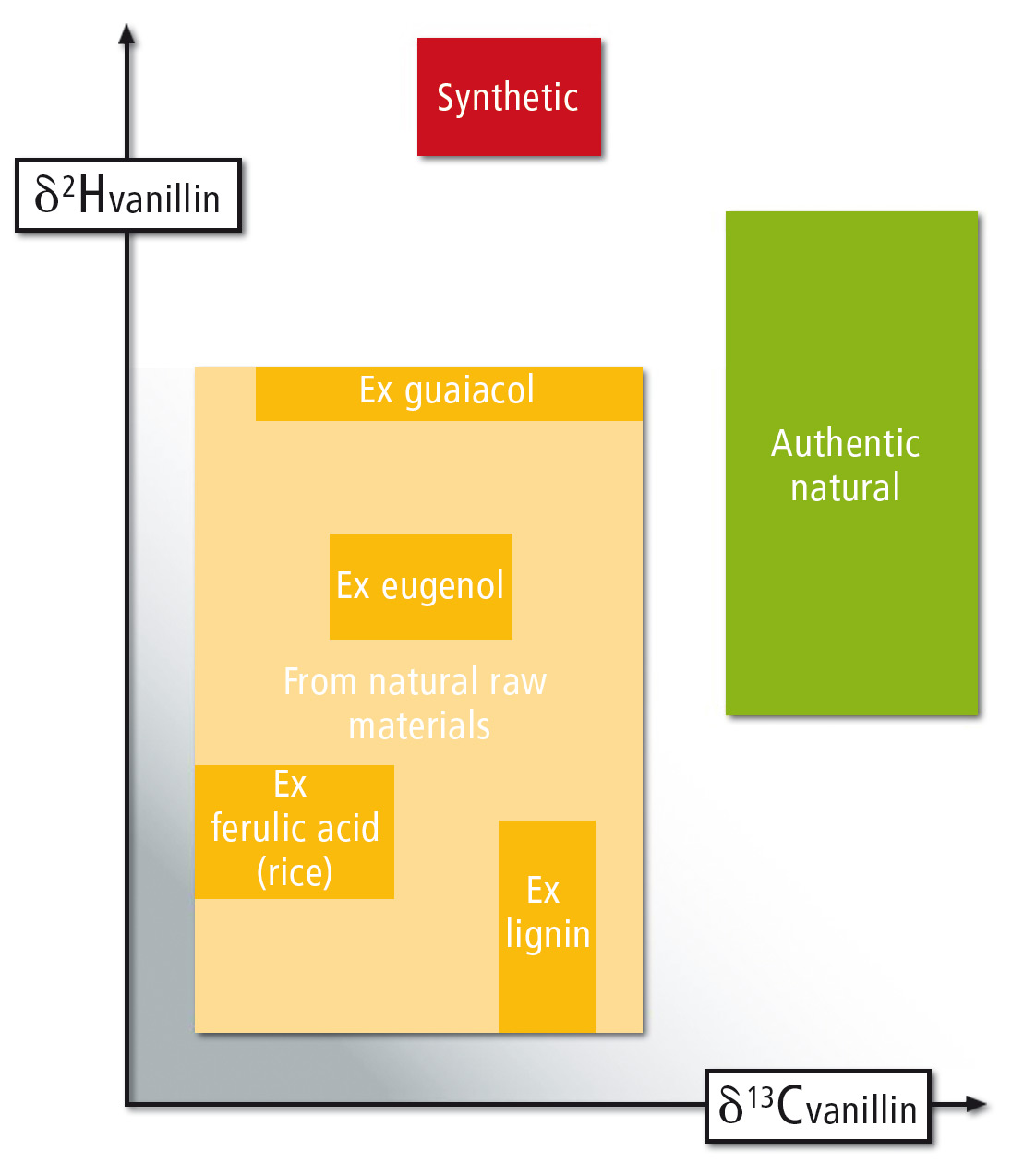 Figure 2: Isotopic signature of vanilla flavour. Possible isotopic signature ranges from natural, biotechnological and synthetic vanillin are shown (data from Imprint Analytics).
Figure 2: Isotopic signature of vanilla flavour. Possible isotopic signature ranges from natural, biotechnological and synthetic vanillin are shown (data from Imprint Analytics).
Outlook
All vanillin sources can be determined using the technology and method described. But new possibilities to produce vanillin in the future by other ways will occur. For this reason, this method should be developed further. Oxygen isotopes should be considered in the future to ensure robust results on the authenticity of vanillin. A third measurement is required, where the oxygen of vanillin reacts to CO in the platinum oven.
A large number of flavor substances are falsified (e.g. benzaldehyde from bitter almond, or cinnamaldehyde in cinnamon), just to mention some. Imprint Analytics will develop more method applications for this field.
Authors
Dr. Balázs Horváth, Dr. David Psomiadis, Dr. Bernd Bodiselitsch
Imprint Analytics GmbH, Werner von Siemens Str. 1, 7343 Neutal, Austria
Literature
[1] Hoffman, P.G., Salb, M. (1979) Isolation and stable isotope ratio analysis of vanillin. J. Agric. Food Chem., 1979, 27 (2), pp 352-355
[2] Greule, M., Tumino, L.D., Kronewald, T., Hener, U., Schleucher, J., Mosandl, A., & Keppler, F. (2010) Improved rapid authentication of vanillin using 13C and 2H values. European Food Research and Technology, 231: 933-41
Further information on this article
• Website: Imprint Analytics