Investigating soil pollution
Analysis of pesticide residues in soils using supercritical fluids
Intensive agriculture and its associated use of pesticides have resulted in the presence of pesticide residues in our entire ecosystem. In 2006 and 2007, about 2.4 megatons of pesticides were used worldwide to protect fruits and vegetables from insect infestation. However, not all crop protection agents act only on targeted pests. Often, beneficial insects are also non-selectively affected.
When resistant insecticides and fungicides get into human and animal foods via water and air, additional health risks arise. These range from simple skin and eye irritation to nervous system damage, hormone-like effects or possibly even certain kinds of cancer.

The ecological footprint of the ideal pesticide
This is why, in the development and regulatory approval of new pesticides, their ecological footprints are essential in terms of stability and decomposition studies. The ideal pesticide should be as selective as possible and target only specific pests. In addition, it should be quickly biodegradable without leaving any residues. As the group of pesticides include many different compound classes, they differ strongly in terms of their chemical structure and characteristics, which makes the analysis of these types of compounds rather complex.
For the determination of pesticide residues in soils, an initial extraction step is required. Typically, this step is carried out using liquid-liquid extraction. This is, however, time-consuming – also due to the use of special reagents and laboratory equipment.
The analysis is often complicated by contamination due to the presence of metal ions or other ionic compounds. Also challenging is the sensitivity of certain pesticides to external influences such as oxidation, exothermic reactions or other effects that result in partial decomposition of the analytes during liquid-liquid extraction.
Supercritical fluids for automated extraction of pesticides from soils
To avoid these problems, extraction using supercritical carbon dioxide (CO2) offers a gentle alternative to the conventional liquid-liquid extraction. Supercritical fluids combine the properties of gases and liquids: They have a low viscosity while exhibiting high diffusivities – just like gases.
However, they also possess a high solubility, just like liquids. CO2 (at or above its critical temperature and critical pressure) is the most commonly used supercritical fluid for chromatographic purposes because it is highly inert, non-toxic and inexpensive, in addition to having suitable physico-chemical properties and being easily available.
Supercritical fluid extraction (SFE) using supercritical CO2 therefore combines the good solubility properties of liquid-liquid extraction with the excellent diffusion properties of gases, so that the sample to be extracted is optimally permeated and the analytes can be extracted efficiently. By using carbon dioxide, the need for large amounts of organic solvents is eliminated, thereby reducing solvent waste.
Extraction of pesticides without the need for sample pretreatment
Shimadzu’s Nexera UC SFE system was used for the extraction of pesticides. The analytical conditions are presented in table 1. In contrast to the traditional liquid-liquid extraction, no complex sample preparation is necessary, except for the addition of a drying agent to bind residual moisture. A typical sample preparation workflow is shown in figure 1.
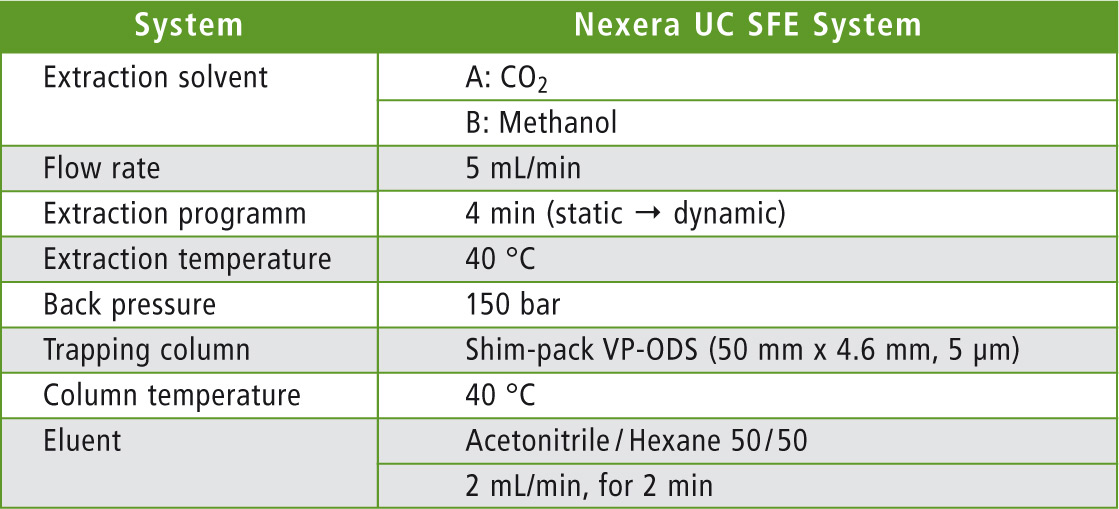 Table 1: Analytical conditions of the extraction
Table 1: Analytical conditions of the extraction
 Figure 1: Sample preparation for SFE
Figure 1: Sample preparation for SFE
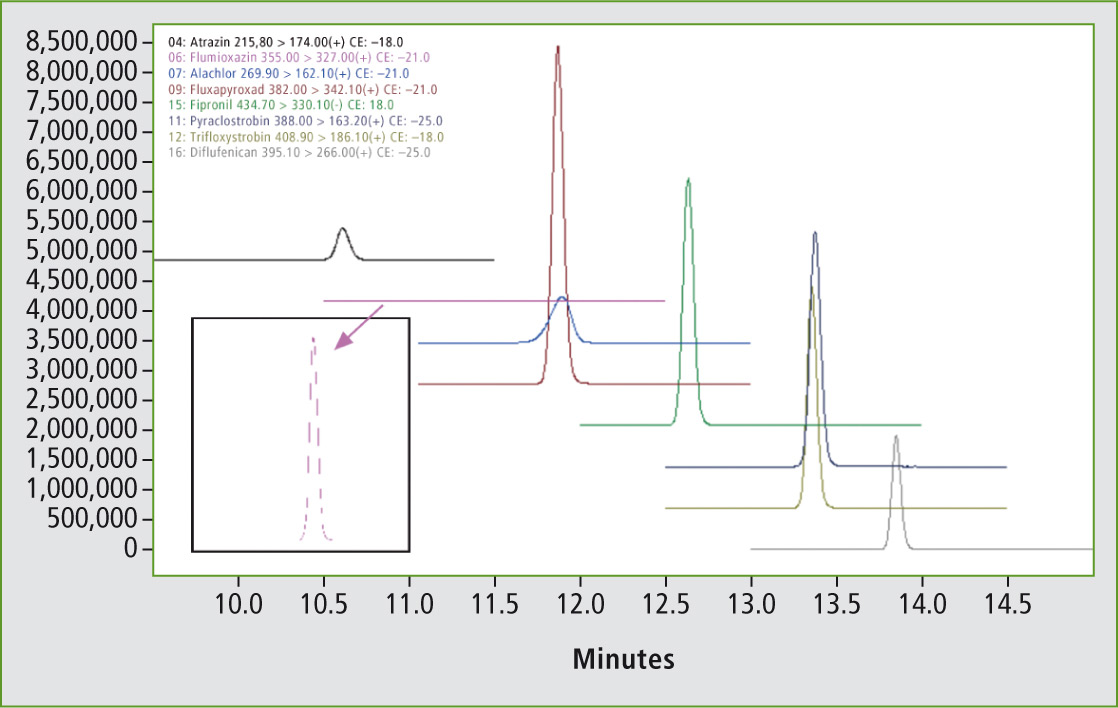 Figure 2: MRM-chromatograms of the pesticides
Figure 2: MRM-chromatograms of the pesticides
By expanding the autosampler with a rack changer, automated preparation and processing of up to 48 samples is possible. In this way, the analysis is not only much more time-efficient and possible overnight or during the weekend but, in addition, human errors during sample preparation are also minimized.
For the actual extraction, the extraction vessels can be heated up to 80 °C. Meanwhile, the trapping column can be rinsed and equilibrated (see figure 3A).
Using supercritical carbon dioxide and optionally additional organic modifiers, the analytes are extracted from the sample and retained on a trapping column (B).
If necessary, further rinsing steps for the purification of the extract on the column can be implemented (C).
The extract is then released from the column by an eluent and collected in the fraction collector (D).
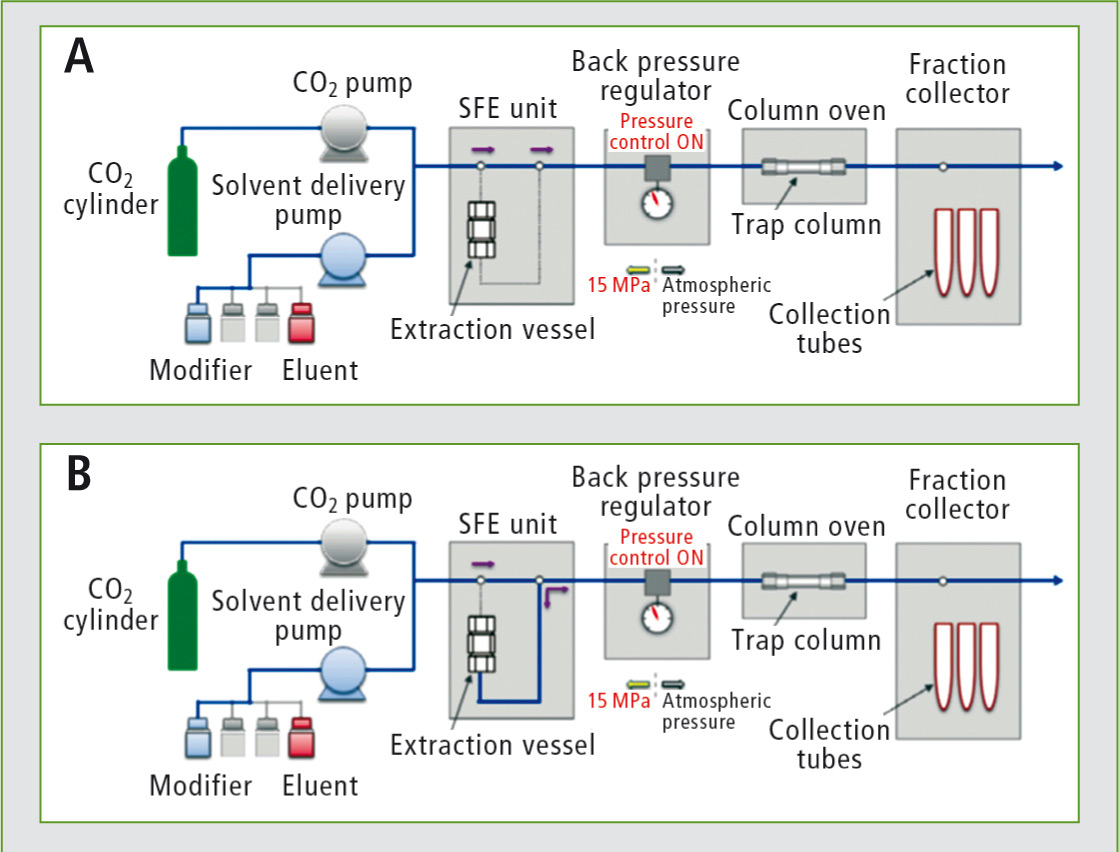
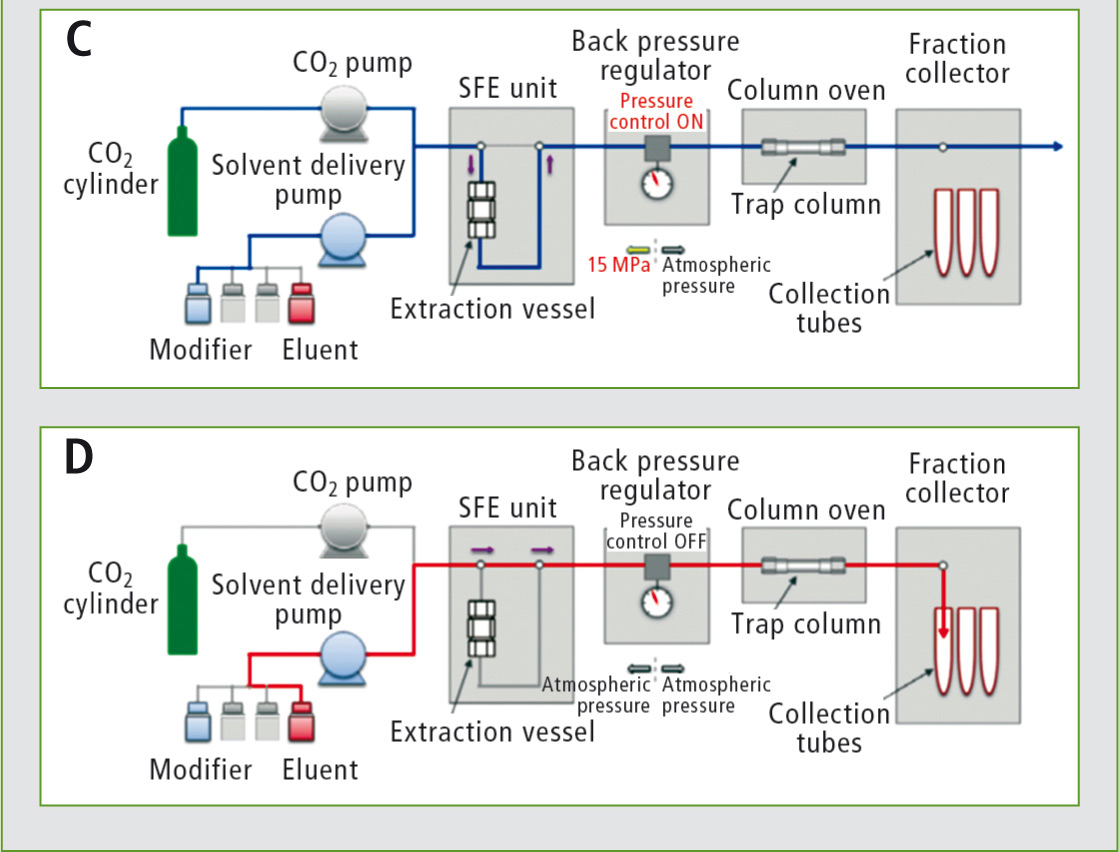 Figure 3: Exemplary scheme of the extraction process
Figure 3: Exemplary scheme of the extraction process
By using the trapping column, multiple extraction steps are possible without diluting the sample unnecessarily, as the release only takes place after addition of the eluent.
Conclusion
Extraction using the Nexera UC demonstrates that the complex sample preparation usually needed for the analysis of pesticides in soil samples can be avoided. The use of supercritical carbon dioxide ensures an efficient extraction and also minimizes the amount of organic solvents used. Autosampler and rack-changer automate the analysis. Their use precludes the influence of human error. In this way, the Nexera UC offers a simple and fast method for sample preparation prior to residue analysis and provides a reliable investigation of pesticide pollution in soil samples.